当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
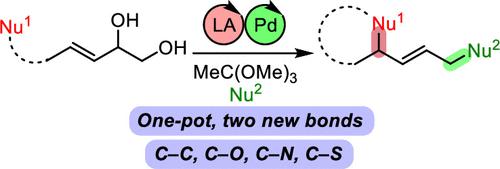
Regio- and Chemoselective Double Allylic Substitution of Alkenyl vic-Diols
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.orglett.4c00495 Bocheng Chen 1 , Lucas Pagès 1 , Raphaël Dollet 1 , Cyrille Kouklovsky 1 , Sébastien Prévost 2 , Aurélien de la Torre 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.orglett.4c00495 Bocheng Chen 1 , Lucas Pagès 1 , Raphaël Dollet 1 , Cyrille Kouklovsky 1 , Sébastien Prévost 2 , Aurélien de la Torre 1
Affiliation
|
Double allylic substitution is an attractive approach to building molecular complexity from simple starting materials by creating two new bonds in one pot. However, this type of reaction has been doomed by chemoselectivity and regioselectivity issues. In this letter, we describe a new approach to introduce a-la-carte two new C–C, C–N, C–O, or C–S bonds in a chemo- and regioselective fashion. The reaction relies on sequential dual catalysis with a Lewis acid and palladium. The scope is remarkably broad, and the reaction can be diastereoselective by using secondary alcohols as the first nucleophile.
中文翻译:

烯基邻二醇的区域和化学选择性双烯丙基取代
双烯丙基取代是一种有吸引力的方法,通过在一个锅中创建两个新键,从简单的起始材料构建分子复杂性。然而,这种类型的反应因化学选择性和区域选择性问题而注定失败。在这封信中,我们描述了一种新方法,以化学和区域选择性的方式引入两个新的 C-C、C-N、C-O 或 C-S 键。该反应依赖于路易斯酸和钯的连续双重催化。范围非常广泛,并且通过使用仲醇作为第一亲核试剂,该反应可以是非对映选择性的。
更新日期:2024-03-15
中文翻译:

烯基邻二醇的区域和化学选择性双烯丙基取代
双烯丙基取代是一种有吸引力的方法,通过在一个锅中创建两个新键,从简单的起始材料构建分子复杂性。然而,这种类型的反应因化学选择性和区域选择性问题而注定失败。在这封信中,我们描述了一种新方法,以化学和区域选择性的方式引入两个新的 C-C、C-N、C-O 或 C-S 键。该反应依赖于路易斯酸和钯的连续双重催化。范围非常广泛,并且通过使用仲醇作为第一亲核试剂,该反应可以是非对映选择性的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号