当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of β‐Aryl‐α,β‐Dehydroaminophosphonates by Pd‐Catalyzed Fujiwara–Moritani C−C Coupling
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-03-16 , DOI: 10.1002/ejoc.202400146 Javier Sáez 1 , David Dalmau 1 , Francisco J. Sayago 1 , Esteban P. Urriolabeitia 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-03-16 , DOI: 10.1002/ejoc.202400146 Javier Sáez 1 , David Dalmau 1 , Francisco J. Sayago 1 , Esteban P. Urriolabeitia 1
Affiliation
|
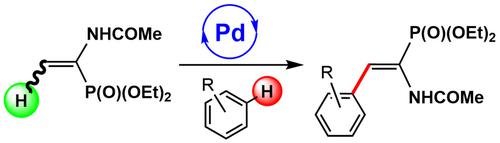
The treatment of diethyl α,β‐dehydroaminophosphonate 1 with various arenes (ArH=toluene 2 a, benzene 2 b, anisole 2 c, bromobenzene 2 d, chlorobenzene 2 e, benzyl alcohol 2 f, p ‐xylene 2 g) in acetic acid (AcOH) at 120 °C for 8 h in the presence of Pd(OAc)2 (10 % mol, OAc=acetate) and AgOAc (3.4 equivalents) results in the formation of the corresponding β‐aryl derivatives E ‐Ar(H)C=C(NHAc)P(O)(OEt)2 3 a–3 g. The reaction proceeds through double C−H activation (arene and alkene) and subsequent C−C oxidative coupling (Fujiwara–Moritani reaction), processes catalyzed by Pd and assisted by Ag. The obtained products 3 a–3 g are isosteric analogs of phenylalanine and are obtained with high selectivity. Thus, geometrical E ‐isomers have been obtained in all studied cases, however mixtures of ortho ‐/meta ‐/para ‐isomers are observed when the activated position in the starting arene 2 is considered.
中文翻译:

Pd 催化 Fujiwara-Moritani C−C 偶联合成 β-芳基-α,β-脱氢氨基膦酸酯
α,β-脱氢氨基膦酸二乙酯 1 用各种芳烃(ArH=甲苯 2a、苯 2b、苯甲醚 2c、溴苯 2d、氯苯 2e、苯甲醇 2f、p -二甲苯 2 g) 在乙酸 (AcOH) 中,在 Pd(OAc) 存在下,在 120 °C 下反应 8 小时2 (10% mol,OAc=乙酸盐)和 AgOAc(3.4 当量)导致形成相应的 β-芳基衍生物乙 ‐Ar(H)C=C(NHAc)P(O)(OEt)2 3 a–3 g。该反应通过双 C−H 活化(芳烃和烯烃)和随后的 C−C 氧化偶联(Fujiwara-Moritani 反应)进行,该过程由 Pd 催化并由 Ag 辅助。所得产物3a-3g是苯丙氨酸的等排类似物,并且以高选择性获得。因此,几何乙 在所有研究案例中都获得了异构体,但是异构体的混合物正射 ‐/元 ‐/帕拉 当考虑起始芳烃 2 中的活化位置时,观察到异构体。
更新日期:2024-03-16
中文翻译:

Pd 催化 Fujiwara-Moritani C−C 偶联合成 β-芳基-α,β-脱氢氨基膦酸酯
α,β-脱氢氨基膦酸二乙酯 1 用各种芳烃(ArH=甲苯 2a、苯 2b、苯甲醚 2c、溴苯 2d、氯苯 2e、苯甲醇 2f、



























 京公网安备 11010802027423号
京公网安备 11010802027423号