当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible light catalyzed arylsilylation of alkenes to construct silicon-containing 1,1-diaryl moieties
Green Chemistry ( IF 9.8 ) Pub Date : 2024-03-15 , DOI: 10.1039/d4gc00073k Jia Cao 1, 2 , Liuzhou Gao 1 , Guoqiang Wang 1 , Shuhua Li 1
Green Chemistry ( IF 9.8 ) Pub Date : 2024-03-15 , DOI: 10.1039/d4gc00073k Jia Cao 1, 2 , Liuzhou Gao 1 , Guoqiang Wang 1 , Shuhua Li 1
Affiliation
|
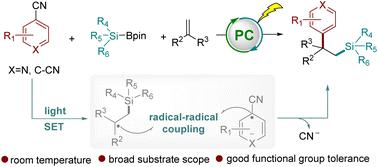
We established an efficient photocatalyzed arylsilylation of alkenes, utilizing readily accessible silylboronates and (hetero)aryl nitriles in the presence of a base. This reaction proceeds through a photoredox-triggered cascade of single-electron transfers of base/silylboronate adducts and (hetero)aryl nitriles with the photocatalyst, leading to the formation of the silyl radicals and (hetero)aryl nitrile radical anions. This is followed by sequential coupling with alkenes, yielding valuable 1,1-diaryl derivatives with a silicon group at the β-position. This protocol features good functional group tolerance, mild reaction conditions, and broad substrate scopes, and thus can be applicable to the structural modification of bioactive molecules.
中文翻译:

可见光催化烯烃芳基硅烷化构建含硅 1,1-二芳基部分
我们在碱存在下利用容易获得的甲硅烷基硼酸酯和(杂)芳基腈,建立了一种有效的烯烃光催化芳基甲硅烷基化反应。该反应通过光氧化还原触发的碱/甲硅烷基硼酸酯加合物和(杂)芳基腈与光催化剂的单电子转移级联进行,导致甲硅烷基自由基和(杂)芳基腈自由基阴离子的形成。随后与烯烃连续偶联,产生有价值的 1,1-二芳基衍生物,其 β 位带有硅基团。该方案具有良好的官能团耐受性、反应条件温和、底物范围广等特点,可适用于生物活性分子的结构修饰。
更新日期:2024-03-20
中文翻译:

可见光催化烯烃芳基硅烷化构建含硅 1,1-二芳基部分
我们在碱存在下利用容易获得的甲硅烷基硼酸酯和(杂)芳基腈,建立了一种有效的烯烃光催化芳基甲硅烷基化反应。该反应通过光氧化还原触发的碱/甲硅烷基硼酸酯加合物和(杂)芳基腈与光催化剂的单电子转移级联进行,导致甲硅烷基自由基和(杂)芳基腈自由基阴离子的形成。随后与烯烃连续偶联,产生有价值的 1,1-二芳基衍生物,其 β 位带有硅基团。该方案具有良好的官能团耐受性、反应条件温和、底物范围广等特点,可适用于生物活性分子的结构修饰。



























 京公网安备 11010802027423号
京公网安备 11010802027423号