当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correction to “Macrocyclic Immunoproteasome Inhibitors as a Potential Therapy for Alzheimer’s Disease”
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-03-12 , DOI: 10.1021/acs.jmedchem.4c00382 Min Jae Lee , Deepak Bhattarai , Hyeryung Jang , Ahreum Baek , In Jun Yeo , Seongsoo Lee , Zachary Miller , Sukyeong Lee , Jin Tae Hong , Dong-Eun Kim , Wooin Lee , Kyung Bo Kim
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-03-12 , DOI: 10.1021/acs.jmedchem.4c00382 Min Jae Lee , Deepak Bhattarai , Hyeryung Jang , Ahreum Baek , In Jun Yeo , Seongsoo Lee , Zachary Miller , Sukyeong Lee , Jin Tae Hong , Dong-Eun Kim , Wooin Lee , Kyung Bo Kim
|
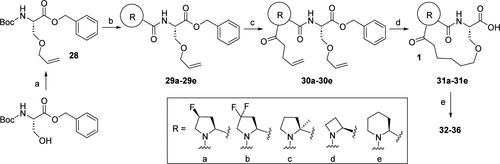
Pages 10936 and 10937. Errors have been identified in Scheme 2, structure a in the box, and Table 1, structure 32 in the box. The stereochemistry of the fluoride group in a and 32 is incorrectly depicted as trans-fluoro (R configuration); the correct stereochemistry is cis-fluoro (S configuration). The corrected Scheme 2 and Table 1 are shown below. aReagents and conditions: (a) allyl methyl carbonate, Pd(PPh3)4, THF, 60 °C, 3 h; (b) TFA, DCM, rt, 1 h, then evaporated and dried, N-Boc-proline derivative, HBTU, HOBt, DIPEA, DCM, rt, 18 h; (c) (1) TFA, DCM, rt, 1 h, then evaporated and dried; (2) alkenyl carboxylic acid, HBTU, HOBt, DIPEA, DCM, rt, 18 h; (d) (1) Grubb’s second-generation catalyst, toluene, 90 °C, 1 h, purified by flash column chromatography; (2) H2, Pd/C, methanol, rt, 1 h; (e) amine-deprotected epoxyketone (4a), HBTU, HOBt, DIPEA, DCM, rt, 18 h. The activity of individual proteasome subunits was measured using purified human 20S proteasomes and the respective fluorogenic substrates, Ac-PAL-AMC (for LMP2), Ac-nLPnLD-AMC (for Y), Ac-ANW-AMC (for LMP7), and Ac-WLA-AMC (for X). Data were obtained based on the results of three replicates per compound. N.D. denotes “not determined”. This article has not yet been cited by other publications. aReagents and conditions: (a) allyl methyl carbonate, Pd(PPh3)4, THF, 60 °C, 3 h; (b) TFA, DCM, rt, 1 h, then evaporated and dried, N-Boc-proline derivative, HBTU, HOBt, DIPEA, DCM, rt, 18 h; (c) (1) TFA, DCM, rt, 1 h, then evaporated and dried; (2) alkenyl carboxylic acid, HBTU, HOBt, DIPEA, DCM, rt, 18 h; (d) (1) Grubb’s second-generation catalyst, toluene, 90 °C, 1 h, purified by flash column chromatography; (2) H2, Pd/C, methanol, rt, 1 h; (e) amine-deprotected epoxyketone (4a), HBTU, HOBt, DIPEA, DCM, rt, 18 h.
中文翻译:

对“大环免疫蛋白酶体抑制剂作为阿尔茨海默病的潜在疗法”的更正
第 10936 页和第 10937 页。方案 2(方框中的结构a)和表 1(方框中的结构32)中已发现错误。 a和32中氟化物基团的立体化学被错误地描述为反式氟( R构型);正确的立体化学是顺式-氟( S构型)。修正后的方案2和表1如下所示。 a试剂和条件:(a)碳酸烯丙酯甲酯,Pd(PPh 3 ) 4 ,THF,60℃,3小时; (b) TFA、DCM,室温1小时,然后蒸发并干燥,N-Boc-脯氨酸衍生物、HBTU、HOBt、DIPEA、DCM,室温18小时; (c) (1) TFA、DCM,室温,1小时,然后蒸发并干燥; (2)烯基羧酸、HBTU、HOBt、DIPEA、DCM,室温,18小时; (d) (1) Grubb第二代催化剂,甲苯,90℃,1h,快速柱层析纯化; (2) H 2 ,Pd/C,甲醇,室温,1小时; (e) 胺去保护的环氧酮( 4a )、HBTU、HOBt、DIPEA、DCM,室温,18小时。使用纯化的人 20S 蛋白酶体和各自的荧光底物 Ac-PAL-AMC(对于 LMP2)、Ac-nLPnLD-AMC(对于 Y)、Ac-ANW-AMC(对于 LMP7)和 Ac-nLPnLD-AMC(对于 LMP7)测量各个蛋白酶体亚基的活性。 Ac-WLA-AMC(对于 X)。数据是根据每种化合物三次重复的结果获得的。 ND 表示“未确定”。这篇文章尚未被其他出版物引用。 a试剂和条件:(a)碳酸烯丙酯甲酯,Pd(PPh 3 ) 4 ,THF,60℃,3小时; (b) TFA、DCM,室温1小时,然后蒸发并干燥,N-Boc-脯氨酸衍生物、HBTU、HOBt、DIPEA、DCM,室温18小时; (c) (1) TFA、DCM,室温,1小时,然后蒸发并干燥; (2)烯基羧酸、HBTU、HOBt、DIPEA、DCM,室温,18小时; (d) (1) Grubb第二代催化剂,甲苯,90℃,1h,快速柱层析纯化; (2) H 2 ,Pd/C,甲醇,室温,1小时; (e) 胺去保护的环氧酮( 4a )、HBTU、HOBt、DIPEA、DCM,室温,18小时。
更新日期:2024-03-12
中文翻译:

对“大环免疫蛋白酶体抑制剂作为阿尔茨海默病的潜在疗法”的更正
第 10936 页和第 10937 页。方案 2(方框中的结构a)和表 1(方框中的结构32)中已发现错误。 a和32中氟化物基团的立体化学被错误地描述为反式氟( R构型);正确的立体化学是顺式-氟( S构型)。修正后的方案2和表1如下所示。 a试剂和条件:(a)碳酸烯丙酯甲酯,Pd(PPh 3 ) 4 ,THF,60℃,3小时; (b) TFA、DCM,室温1小时,然后蒸发并干燥,N-Boc-脯氨酸衍生物、HBTU、HOBt、DIPEA、DCM,室温18小时; (c) (1) TFA、DCM,室温,1小时,然后蒸发并干燥; (2)烯基羧酸、HBTU、HOBt、DIPEA、DCM,室温,18小时; (d) (1) Grubb第二代催化剂,甲苯,90℃,1h,快速柱层析纯化; (2) H 2 ,Pd/C,甲醇,室温,1小时; (e) 胺去保护的环氧酮( 4a )、HBTU、HOBt、DIPEA、DCM,室温,18小时。使用纯化的人 20S 蛋白酶体和各自的荧光底物 Ac-PAL-AMC(对于 LMP2)、Ac-nLPnLD-AMC(对于 Y)、Ac-ANW-AMC(对于 LMP7)和 Ac-nLPnLD-AMC(对于 LMP7)测量各个蛋白酶体亚基的活性。 Ac-WLA-AMC(对于 X)。数据是根据每种化合物三次重复的结果获得的。 ND 表示“未确定”。这篇文章尚未被其他出版物引用。 a试剂和条件:(a)碳酸烯丙酯甲酯,Pd(PPh 3 ) 4 ,THF,60℃,3小时; (b) TFA、DCM,室温1小时,然后蒸发并干燥,N-Boc-脯氨酸衍生物、HBTU、HOBt、DIPEA、DCM,室温18小时; (c) (1) TFA、DCM,室温,1小时,然后蒸发并干燥; (2)烯基羧酸、HBTU、HOBt、DIPEA、DCM,室温,18小时; (d) (1) Grubb第二代催化剂,甲苯,90℃,1h,快速柱层析纯化; (2) H 2 ,Pd/C,甲醇,室温,1小时; (e) 胺去保护的环氧酮( 4a )、HBTU、HOBt、DIPEA、DCM,室温,18小时。











































 京公网安备 11010802027423号
京公网安备 11010802027423号