当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism-Based Macrocyclic Inhibitors of Serine Proteases
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2024-03-13 , DOI: 10.1021/acs.jmedchem.3c02388 Vishnu C. Damalanka 1 , Victoria Banas 1 , Paolo De Bona 1 , Maithri M. Kashipathy 2 , Kevin Battaile 3 , Scott Lovell 2 , James W. Janetka 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2024-03-13 , DOI: 10.1021/acs.jmedchem.3c02388 Vishnu C. Damalanka 1 , Victoria Banas 1 , Paolo De Bona 1 , Maithri M. Kashipathy 2 , Kevin Battaile 3 , Scott Lovell 2 , James W. Janetka 1
Affiliation
|
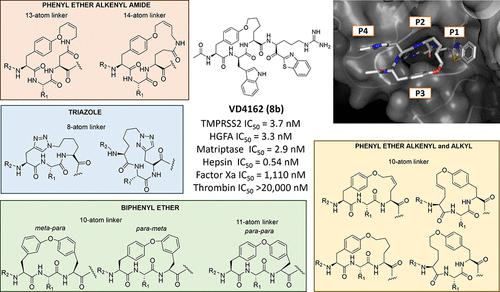
Protease inhibitor drug discovery is challenged by the lack of cellular and oral permeability, selectivity, metabolic stability, and rapid clearance of peptides. Here, we describe the rational design, synthesis, and evaluation of peptidomimetic side-chain-cyclized macrocycles which we converted into covalent serine protease inhibitors with the addition of an electrophilic ketone warhead. We have identified potent and selective inhibitors of TMPRSS2, matriptase, hepsin, and HGFA and demonstrated their improved protease selectivity, metabolic stability, and pharmacokinetic (PK) properties. We obtained an X-ray crystal structure of phenyl ether-cyclized tripeptide VD4162 (8b) bound to matriptase, revealing an unexpected binding conformation. Cyclic biphenyl ether VD5123 (11) displayed the best PK properties in mice with a half-life of 4.5 h and compound exposure beyond 24 h. These new cyclic tripeptide scaffolds can be used as easily modifiable templates providing a new strategy to overcoming the obstacles presented by linear acyclic peptides in protease inhibitor drug discovery.
中文翻译:

基于机制的丝氨酸蛋白酶大环抑制剂
蛋白酶抑制剂药物的发现面临着肽缺乏细胞和口腔渗透性、选择性、代谢稳定性和快速清除的挑战。在这里,我们描述了拟肽侧链环化大环化合物的合理设计、合成和评估,我们通过添加亲电子酮弹头将其转化为共价丝氨酸蛋白酶抑制剂。我们已经鉴定出 TMPRSS2、matriptase、hepsin 和 HGFA 的有效选择性抑制剂,并证明了它们改善的蛋白酶选择性、代谢稳定性和药代动力学 (PK) 特性。我们获得了与 matriptase 结合的苯醚环化三肽 VD4162 ( 8b ) 的 X 射线晶体结构,揭示了意想不到的结合构象。环联苯醚 VD5123 ( 11 ) 在小鼠中表现出最佳的 PK 特性,半衰期为 4.5 小时,化合物暴露时间超过 24 小时。这些新的环状三肽支架可用作易于修改的模板,为克服蛋白酶抑制剂药物发现中线性无环肽所带来的障碍提供了新的策略。
更新日期:2024-03-13
中文翻译:

基于机制的丝氨酸蛋白酶大环抑制剂
蛋白酶抑制剂药物的发现面临着肽缺乏细胞和口腔渗透性、选择性、代谢稳定性和快速清除的挑战。在这里,我们描述了拟肽侧链环化大环化合物的合理设计、合成和评估,我们通过添加亲电子酮弹头将其转化为共价丝氨酸蛋白酶抑制剂。我们已经鉴定出 TMPRSS2、matriptase、hepsin 和 HGFA 的有效选择性抑制剂,并证明了它们改善的蛋白酶选择性、代谢稳定性和药代动力学 (PK) 特性。我们获得了与 matriptase 结合的苯醚环化三肽 VD4162 ( 8b ) 的 X 射线晶体结构,揭示了意想不到的结合构象。环联苯醚 VD5123 ( 11 ) 在小鼠中表现出最佳的 PK 特性,半衰期为 4.5 小时,化合物暴露时间超过 24 小时。这些新的环状三肽支架可用作易于修改的模板,为克服蛋白酶抑制剂药物发现中线性无环肽所带来的障碍提供了新的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号