当前位置:
X-MOL 学术
›
Am. J. Obstet. Gynecol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metformin in pregnancy and childhood neurodevelopmental outcomes: a systematic review and meta-analysis
American Journal of Obstetrics and Gynecology ( IF 9.8 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.ajog.2024.02.316 Hannah G. Gordon , Jessica A. Atkinson , Stephen Tong , Parinaz Mehdipour , Catherine Cluver , Susan P. Walker , Anthea C. Lindquist , Roxanne M. Hastie
American Journal of Obstetrics and Gynecology ( IF 9.8 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.ajog.2024.02.316 Hannah G. Gordon , Jessica A. Atkinson , Stephen Tong , Parinaz Mehdipour , Catherine Cluver , Susan P. Walker , Anthea C. Lindquist , Roxanne M. Hastie
|
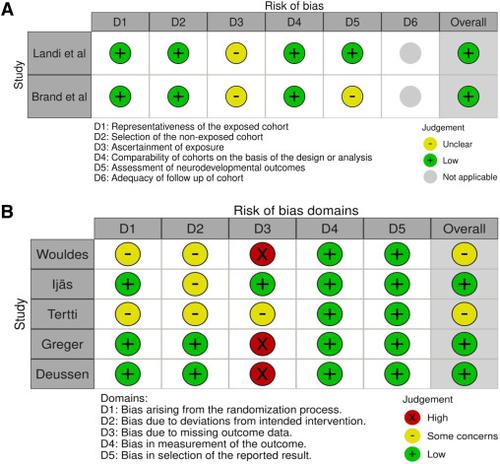
This study aimed to examine the impact of maternal metformin use during pregnancy on offspring neurodevelopmental outcomes. MEDLINE, Embase, and Web of Science (Core Collection) were searched from inception until July 1, 2023. Studies of women who received treatment with metformin at any stage of pregnancy for any indication with neurodevelopmental data available for their offspring were included. Studies without a control group were excluded. Randomized controlled trials, case-control, cohort, and cross-sectional studies were included in the review. Studies were screened for inclusion and data were extracted independently by 2 reviewers. Risk of bias was assessed using a modified version of the Newcastle-Ottawa Scale for nonrandomized studies, and the Risk of Bias 2 tool for randomized trials. A total of 7 studies met the inclusion criteria, including a combined cohort of 14,042 children with 7641 children who were exposed and followed for up to 14 years of age. Metformin use during pregnancy was not associated with neurodevelopmental delay in infancy (relative risk, 1.09; 95% confidence interval, 0.54–2.17; 3 studies; 9668 children) or at ages 3 to 5 years (relative risk, 0.90; 95% confidence interval, 0.56–1.45; 2 studies; 6118 children). When compared with unexposed peers, metformin use during pregnancy was not associated with altered motor scores (mean difference, 0.30; 95% confidence interval, −1.15 to 1.74; 3 studies; 714 children) or cognitive scores (mean difference, −0.45; 95% confidence interval, −1.45 to 0.55; 4 studies; 734 children). Studies that were included were of high quality and deemed to be at low risk of bias. In utero exposure to metformin does not seem to be associated with adverse neurodevelopmental outcomes in children up to the age of 14 years. These findings provide reassurance to clinicians and pregnant women considering metformin use during pregnancy.
中文翻译:

二甲双胍在妊娠和儿童神经发育结局中的作用:系统评价和荟萃分析
本研究旨在探讨母亲在怀孕期间使用二甲双胍对后代神经发育结果的影响。 MEDLINE、Embase 和 Web of Science(核心合集)的检索时间从最初开始一直到 2023 年 7 月 1 日。纳入了对在怀孕任何阶段接受二甲双胍治疗的女性的研究,以及其后代可获得的神经发育数据。没有对照组的研究被排除在外。该评价包括随机对照试验、病例对照、队列和横断面研究。研究进行了纳入筛选,数据由 2 名评审员独立提取。对于非随机研究,使用改良版纽卡斯尔-渥太华量表评估偏倚风险;对于随机试验,使用偏倚风险 2 工具进行评估。共有 7 项研究符合纳入标准,其中包括 14,042 名儿童和 7641 名接受过暴露并随访至 14 岁的儿童。怀孕期间使用二甲双胍与婴儿期(相对风险,1.09;95% 置信区间,0.54–2.17;3 项研究;9668 名儿童)或 3 至 5 岁时(相对风险,0.90;95% 置信区间)神经发育迟缓无关。 ,0.56–1.45;2 项研究;6118 名儿童)。与未接触过二甲双胍的同龄人相比,怀孕期间使用二甲双胍与运动评分的改变(平均差,0.30;95%置信区间,-1.15至1.74;3项研究;714名儿童)或认知评分(平均差,-0.45;95)无关。 % 置信区间,-1.45 至 0.55;4 项研究;734 名儿童)。纳入的研究质量很高,且偏倚风险较低。对于 14 岁以下儿童,子宫内接触二甲双胍似乎与神经发育不良结果无关。这些发现让临床医生和考虑在怀孕期间使用二甲双胍的孕妇放心。
更新日期:2024-03-07
中文翻译:

二甲双胍在妊娠和儿童神经发育结局中的作用:系统评价和荟萃分析
本研究旨在探讨母亲在怀孕期间使用二甲双胍对后代神经发育结果的影响。 MEDLINE、Embase 和 Web of Science(核心合集)的检索时间从最初开始一直到 2023 年 7 月 1 日。纳入了对在怀孕任何阶段接受二甲双胍治疗的女性的研究,以及其后代可获得的神经发育数据。没有对照组的研究被排除在外。该评价包括随机对照试验、病例对照、队列和横断面研究。研究进行了纳入筛选,数据由 2 名评审员独立提取。对于非随机研究,使用改良版纽卡斯尔-渥太华量表评估偏倚风险;对于随机试验,使用偏倚风险 2 工具进行评估。共有 7 项研究符合纳入标准,其中包括 14,042 名儿童和 7641 名接受过暴露并随访至 14 岁的儿童。怀孕期间使用二甲双胍与婴儿期(相对风险,1.09;95% 置信区间,0.54–2.17;3 项研究;9668 名儿童)或 3 至 5 岁时(相对风险,0.90;95% 置信区间)神经发育迟缓无关。 ,0.56–1.45;2 项研究;6118 名儿童)。与未接触过二甲双胍的同龄人相比,怀孕期间使用二甲双胍与运动评分的改变(平均差,0.30;95%置信区间,-1.15至1.74;3项研究;714名儿童)或认知评分(平均差,-0.45;95)无关。 % 置信区间,-1.45 至 0.55;4 项研究;734 名儿童)。纳入的研究质量很高,且偏倚风险较低。对于 14 岁以下儿童,子宫内接触二甲双胍似乎与神经发育不良结果无关。这些发现让临床医生和考虑在怀孕期间使用二甲双胍的孕妇放心。



























 京公网安备 11010802027423号
京公网安备 11010802027423号