当前位置:
X-MOL 学术
›
Electrochem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of the effect of surface modification with organosilane on the multi-redox electrochemical behavior of Co3[Co(CN)6]2 nanocubes
Electrochemistry Communications ( IF 5.4 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.elecom.2024.107696 Anderson F.M. dos Santos , Lucyano J.A. Macedo , Everson T.S. Gerôncio , Roberto A.S. Luz , Welter Cantanhêde
Electrochemistry Communications ( IF 5.4 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.elecom.2024.107696 Anderson F.M. dos Santos , Lucyano J.A. Macedo , Everson T.S. Gerôncio , Roberto A.S. Luz , Welter Cantanhêde
|
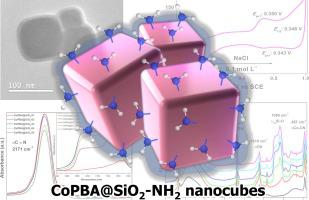
The search for new functional nanomaterials rounds science for decades and supramolecular chemistry is an approach used to properly achieve this goal. The present work reports the successful synthesis of Co[Co(CN)]@SiO-NH nanomaterial, formed from the supramolecular association of cobalt Prussian blue analogue, Co[Co(CN)], and an external amorphous layer (@SiO-NH) originated from the addition of organosilanes as tetraethyl orthosilicate (TEOS) and 3-aminopropyltrimethoxysilane (APTMS). The amount of tetraethyl orthosilicate (TEOS) (0.22, 0.33, 0.44, 0.55, and 0.67 nmol) used to prepare SiO layer was varied to assess its impact on the properties of the new synthesized nanomaterial, which were named Co[Co(CN)]@SiO-NH-01 to Co[Co(CN)]@SiO-NH-05. XRD analyses were consistent with the pattern for Co[Co(CN)]. FTIR measurements confirmed the modification of Co[Co(CN)] nanocubes surface by the formation of SiO, and the attachment of NH groups after the APTMS addition was confirmed by the ninhydrin test, since the UV–Vis analysis showed a band around 576 nm, typical of a Ruherman’s purple. TEM images showed Co[Co(CN)] nanocubes with averaging size around 110 nm, and an amorphous SiO layer around 15 nm thick after modification by TEOS and APTMS. Cyclic voltammetry analyses of Co[Co(CN)]@SiO-NH nanomaterials showed the appearance of second redox process in lower scan rates (10–50 mV s), as the surface of Co[Co(CN)] is modified by TEOS and APTMS. Beyond that, electron transfer processes were evident even with the dielectric coating of SiO at high scan rates, which makes it suitable for application as an electrochemical biosensor as NH-modified-SiO is capable of being functionalized with a wide range of molecules.
中文翻译:

有机硅烷表面修饰对Co3[Co(CN)6]2纳米立方体多重氧化还原电化学行为的影响研究
几十年来,科学界一直在寻找新的功能性纳米材料,超分子化学是实现这一目标的一种方法。目前的工作报告成功合成了 Co[Co(CN)]@SiO-NH 纳米材料,该材料由钴普鲁士蓝类似物 Co[Co(CN)] 和外部非晶层 (@SiO-NH) 的超分子缔合形成)源自添加有机硅烷,如原硅酸四乙酯 (TEOS) 和 3-氨基丙基三甲氧基硅烷 (APTMS)。改变用于制备 SiO2 层的原硅酸四乙酯 (TEOS)(0.22、0.33、0.44、0.55 和 0.67 nmol)的量,以评估其对新合成纳米材料性能的影响,该材料被命名为 Co[Co(CN) ]@SiO-NH-01 至 Co[Co(CN)]@SiO-NH-05。 XRD 分析与 Co[Co(CN)] 的模式一致。 FTIR 测量证实了通过形成 SiO 而对 Co[Co(CN)] 纳米立方体表面进行了修饰,并且通过茚三酮测试证实了添加 APTMS 后 NH 基团的附着,因为 UV-Vis 分析显示在 576 nm 附近有一个谱带,典型的鲁尔曼紫色。 TEM 图像显示,经 TEOS 和 APTMS 改性后,Co[Co(CN)] 纳米立方体平均尺寸约为 110 nm,无定形 SiO 层厚度约为 15 nm。 Co[Co(CN)]@SiO-NH纳米材料的循环伏安分析表明,由于Co[Co(CN)]表面被TEOS修饰,在较低扫描速率(10-50 mV s)下出现了第二次氧化还原过程和 APTMS。除此之外,即使在高扫描速率下使用 SiO 介电涂层,电子转移过程也很明显,这使得它适合用作电化学生物传感器,因为 NH 修饰的 SiO 能够用多种分子进行功能化。
更新日期:2024-03-11
中文翻译:

有机硅烷表面修饰对Co3[Co(CN)6]2纳米立方体多重氧化还原电化学行为的影响研究
几十年来,科学界一直在寻找新的功能性纳米材料,超分子化学是实现这一目标的一种方法。目前的工作报告成功合成了 Co[Co(CN)]@SiO-NH 纳米材料,该材料由钴普鲁士蓝类似物 Co[Co(CN)] 和外部非晶层 (@SiO-NH) 的超分子缔合形成)源自添加有机硅烷,如原硅酸四乙酯 (TEOS) 和 3-氨基丙基三甲氧基硅烷 (APTMS)。改变用于制备 SiO2 层的原硅酸四乙酯 (TEOS)(0.22、0.33、0.44、0.55 和 0.67 nmol)的量,以评估其对新合成纳米材料性能的影响,该材料被命名为 Co[Co(CN) ]@SiO-NH-01 至 Co[Co(CN)]@SiO-NH-05。 XRD 分析与 Co[Co(CN)] 的模式一致。 FTIR 测量证实了通过形成 SiO 而对 Co[Co(CN)] 纳米立方体表面进行了修饰,并且通过茚三酮测试证实了添加 APTMS 后 NH 基团的附着,因为 UV-Vis 分析显示在 576 nm 附近有一个谱带,典型的鲁尔曼紫色。 TEM 图像显示,经 TEOS 和 APTMS 改性后,Co[Co(CN)] 纳米立方体平均尺寸约为 110 nm,无定形 SiO 层厚度约为 15 nm。 Co[Co(CN)]@SiO-NH纳米材料的循环伏安分析表明,由于Co[Co(CN)]表面被TEOS修饰,在较低扫描速率(10-50 mV s)下出现了第二次氧化还原过程和 APTMS。除此之外,即使在高扫描速率下使用 SiO 介电涂层,电子转移过程也很明显,这使得它适合用作电化学生物传感器,因为 NH 修饰的 SiO 能够用多种分子进行功能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号