当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
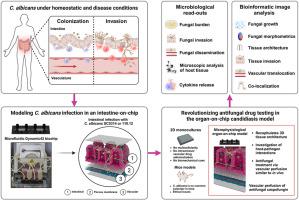
Modeling of intravenous caspofungin administration using an intestine-on-chip reveals altered Candida albicans microcolonies and pathogenicity
Biomaterials ( IF 14.0 ) Pub Date : 2024-03-09 , DOI: 10.1016/j.biomaterials.2024.122525 Tim Kaden , Raquel Alonso-Roman , Parastoo Akbarimoghaddam , Alexander S. Mosig , Katja Graf , Martin Raasch , Bianca Hoffmann , Marc T. Figge , Bernhard Hube , Mark S. Gresnigt
Biomaterials ( IF 14.0 ) Pub Date : 2024-03-09 , DOI: 10.1016/j.biomaterials.2024.122525 Tim Kaden , Raquel Alonso-Roman , Parastoo Akbarimoghaddam , Alexander S. Mosig , Katja Graf , Martin Raasch , Bianca Hoffmann , Marc T. Figge , Bernhard Hube , Mark S. Gresnigt
|
is a commensal yeast of the human intestinal microbiota that, under predisposing conditions, can become pathogenic and cause life-threatening systemic infections (candidiasis). Fungal-host interactions during candidiasis are commonly studied using conventional 2D models, which have provided critical insights into the pathogenicity. However, microphysiological models with a higher biological complexity may be more suitable to mimic -like infection processes and antifungal drug efficacy. Therefore, a 3D intestine-on-chip model was used to investigate fungal-host interactions during the onset of invasive candidiasis and evaluate antifungal treatment under clinically relevant conditions. By combining microbiological and image-based analyses we quantified infection processes such as invasiveness and fungal translocation across the epithelial barrier. Additionally, we obtained novel insights into fungal microcolony morphology and association with the tissue. Our results demonstrate that microcolonies induce injury to the epithelial tissue by disrupting apical cell-cell contacts and causing inflammation. Caspofungin treatment effectively reduced the fungal biomass and induced substantial alterations in microcolony morphology during infection with a wild-type strain. However, caspofungin showed limited effects after infection with an echinocandin-resistant clinical isolate. Collectively, this organ-on-chip model can be leveraged for in-depth characterization of pathogen-host interactions and alterations due to antimicrobial treatment.
中文翻译:

使用肠芯片进行静脉内卡泊芬净给药建模揭示了白色念珠菌微菌落和致病性的改变
是人类肠道微生物群的共生酵母,在诱发条件下,可致病并导致危及生命的全身感染(念珠菌病)。念珠菌病期间真菌与宿主的相互作用通常使用传统的二维模型进行研究,这为致病性提供了重要的见解。然而,具有更高生物复杂性的微生理学模型可能更适合模拟类感染过程和抗真菌药物功效。因此,使用 3D 肠芯片模型来研究侵袭性念珠菌病发作期间的真菌与宿主的相互作用,并评估临床相关条件下的抗真菌治疗。通过结合微生物学和基于图像的分析,我们量化了感染过程,例如侵入性和跨上皮屏障的真菌易位。此外,我们还获得了关于真菌微菌落形态及其与组织关联的新见解。我们的结果表明,微菌落通过破坏顶端细胞-细胞接触并引起炎症来诱导上皮组织损伤。卡泊芬净处理有效地减少了真菌生物量,并在野生型菌株感染期间诱导了微菌落形态的显着改变。然而,卡泊芬净在感染棘白菌素耐药的临床分离株后显示出有限的效果。总的来说,这种器官芯片模型可用于深入表征病原体与宿主的相互作用以及抗菌治疗引起的变化。
更新日期:2024-03-09
中文翻译:

使用肠芯片进行静脉内卡泊芬净给药建模揭示了白色念珠菌微菌落和致病性的改变
是人类肠道微生物群的共生酵母,在诱发条件下,可致病并导致危及生命的全身感染(念珠菌病)。念珠菌病期间真菌与宿主的相互作用通常使用传统的二维模型进行研究,这为致病性提供了重要的见解。然而,具有更高生物复杂性的微生理学模型可能更适合模拟类感染过程和抗真菌药物功效。因此,使用 3D 肠芯片模型来研究侵袭性念珠菌病发作期间的真菌与宿主的相互作用,并评估临床相关条件下的抗真菌治疗。通过结合微生物学和基于图像的分析,我们量化了感染过程,例如侵入性和跨上皮屏障的真菌易位。此外,我们还获得了关于真菌微菌落形态及其与组织关联的新见解。我们的结果表明,微菌落通过破坏顶端细胞-细胞接触并引起炎症来诱导上皮组织损伤。卡泊芬净处理有效地减少了真菌生物量,并在野生型菌株感染期间诱导了微菌落形态的显着改变。然而,卡泊芬净在感染棘白菌素耐药的临床分离株后显示出有限的效果。总的来说,这种器官芯片模型可用于深入表征病原体与宿主的相互作用以及抗菌治疗引起的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号