当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical synthesis of site-selective advanced glycation end products in α-synuclein and its fragments
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2024-03-08 , DOI: 10.1039/d4ob00225c Clara Bosbach 1, 2 , Luisa Maria Gatzemeier 1, 2 , Katja Ilme Bloch von Blottnitz 1 , Annekatrin König 2 , Ulf Diederichsen 1 , Claudia Steinem 1 , Tiago Fleming Outeiro 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2024-03-08 , DOI: 10.1039/d4ob00225c Clara Bosbach 1, 2 , Luisa Maria Gatzemeier 1, 2 , Katja Ilme Bloch von Blottnitz 1 , Annekatrin König 2 , Ulf Diederichsen 1 , Claudia Steinem 1 , Tiago Fleming Outeiro 2, 3, 4
Affiliation
|
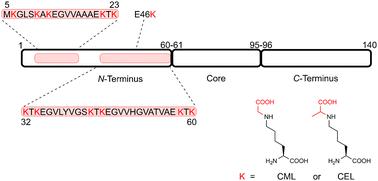
Advanced glycation end products (AGEs) arise from the Maillard reaction between dicarbonyls and proteins, nucleic acids, or specific lipids. Notably, AGEs are linked to aging and implicated in various disorders, spanning from cancer to neurodegenerative diseases. While dicarbonyls like methylglyoxal preferentially target arginine residues, lysine-derived AGEs, such as N(6)-(1-carboxymethyl)lysine (CML) and N(6)-(1-carboxyethyl)lysine (CEL), are also abundant. Predicting protein glycation in vivo proves challenging due to the intricate nature of glycation reactions. In vitro, glycation is difficult to control, especially in proteins that harbor multiple glycation-prone amino acids. α-Synuclein (aSyn), pivotal in Parkinson's disease and synucleinopathies, has 15 lysine residues and is known to become glycated at multiple lysine sites. To understand the influence of glycation in specific regions of aSyn on its behavior, a strategy for site-specific glycated protein production is imperative. To fulfill this demand, we devised a synthetic route integrating solid-phase peptide synthesis, orthogonal protection of amino acid side-chain functionalities, and reductive amination strategies. This methodology yielded two disease-related N-terminal peptide fragments, each featuring five and six CML and CEL modifications, alongside a full-length aSyn protein containing a site-selective E46CEL modification. Our synthetic approach facilitates the broad introduction of glycation motifs at specific sites, providing a foundation for generating glycated forms of synucleinopathy-related and other disease-relevant proteins.
中文翻译:

α-突触核蛋白及其片段中位点选择性高级糖基化终产物的化学合成
晚期糖基化终产物 (AGE) 由二羰基与蛋白质、核酸或特定脂质之间的美拉德反应产生。值得注意的是,AGEs 与衰老有关,并与从癌症到神经退行性疾病等各种疾病有关。虽然甲基乙二醛等二羰基优先靶向精氨酸残基,但赖氨酸衍生的 AGE,例如N (6)-(1-羧甲基) 赖氨酸 (CML) 和N (6)-(1-羧乙基) 赖氨酸 (CEL),也很丰富。由于糖化反应的复杂性,预测体内蛋白质糖化具有挑战性。在体外,糖化很难控制,特别是在含有多个易糖化氨基酸的蛋白质中。α-突触核蛋白 (aSyn) 在帕金森病和突触核蛋白病中发挥着关键作用,具有 15 个赖氨酸残基,已知会在多个赖氨酸位点发生糖化。为了了解 aSyn 特定区域的糖化对其行为的影响,位点特异性糖化蛋白生产的策略势在必行。为了满足这一需求,我们设计了一种集固相肽合成、氨基酸侧链官能团正交保护和还原胺化策略于一体的合成路线。该方法产生了两个与疾病相关的 N 端肽片段,每个片段具有 5 个和 6 个 CML 和 CEL 修饰,以及包含位点选择性 E46CEL 修饰的全长 aSyn 蛋白。我们的合成方法有利于在特定位点广泛引入糖化基序,为生成糖化形式的突触核蛋白病相关蛋白和其他疾病相关蛋白奠定了基础。
更新日期:2024-03-08
中文翻译:

α-突触核蛋白及其片段中位点选择性高级糖基化终产物的化学合成
晚期糖基化终产物 (AGE) 由二羰基与蛋白质、核酸或特定脂质之间的美拉德反应产生。值得注意的是,AGEs 与衰老有关,并与从癌症到神经退行性疾病等各种疾病有关。虽然甲基乙二醛等二羰基优先靶向精氨酸残基,但赖氨酸衍生的 AGE,例如N (6)-(1-羧甲基) 赖氨酸 (CML) 和N (6)-(1-羧乙基) 赖氨酸 (CEL),也很丰富。由于糖化反应的复杂性,预测体内蛋白质糖化具有挑战性。在体外,糖化很难控制,特别是在含有多个易糖化氨基酸的蛋白质中。α-突触核蛋白 (aSyn) 在帕金森病和突触核蛋白病中发挥着关键作用,具有 15 个赖氨酸残基,已知会在多个赖氨酸位点发生糖化。为了了解 aSyn 特定区域的糖化对其行为的影响,位点特异性糖化蛋白生产的策略势在必行。为了满足这一需求,我们设计了一种集固相肽合成、氨基酸侧链官能团正交保护和还原胺化策略于一体的合成路线。该方法产生了两个与疾病相关的 N 端肽片段,每个片段具有 5 个和 6 个 CML 和 CEL 修饰,以及包含位点选择性 E46CEL 修饰的全长 aSyn 蛋白。我们的合成方法有利于在特定位点广泛引入糖化基序,为生成糖化形式的突触核蛋白病相关蛋白和其他疾病相关蛋白奠定了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号