当前位置:
X-MOL 学术
›
Cell Calcium
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recognition of granulocyte-macrophage colony-stimulating factor by specific S100 proteins
Cell Calcium ( IF 4 ) Pub Date : 2024-03-05 , DOI: 10.1016/j.ceca.2024.102869 Alexey S. Kazakov , Victoria A. Rastrygina , Alisa A. Vologzhannikova , Marina Y. Zemskova , Lolita A. Bobrova , Evgenia I. Deryusheva , Maria E. Permyakova , Andrey S. Sokolov , Ekaterina A. Litus , Marina P. Shevelyova , Vladimir N. Uversky , Eugene A. Permyakov , Sergei E. Permyakov
Cell Calcium ( IF 4 ) Pub Date : 2024-03-05 , DOI: 10.1016/j.ceca.2024.102869 Alexey S. Kazakov , Victoria A. Rastrygina , Alisa A. Vologzhannikova , Marina Y. Zemskova , Lolita A. Bobrova , Evgenia I. Deryusheva , Maria E. Permyakova , Andrey S. Sokolov , Ekaterina A. Litus , Marina P. Shevelyova , Vladimir N. Uversky , Eugene A. Permyakov , Sergei E. Permyakov
|
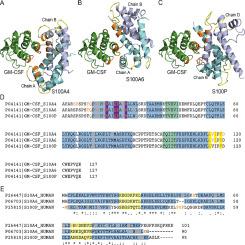
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a pleiotropic myelopoietic growth factor and proinflammatory cytokine, clinically used for multiple indications and serving as a promising target for treatment of many disorders, including cancer, multiple sclerosis, rheumatoid arthritis, psoriasis, asthma, COVID-19. We have previously shown that dimeric Ca-bound forms of S100A6 and S100P proteins, members of the multifunctional S100 protein family, are specific to GM-CSF. To probe selectivity of these interactions, the affinity of recombinant human GM-CSF to dimeric Ca-loaded forms of 18 recombinant human S100 proteins was studied by surface plasmon resonance spectroscopy. Of them, only S100A4 protein specifically binds to GM-CSF with equilibrium dissociation constant, , values of 0.3–2 μM, as confirmed by intrinsic fluorescence and chemical crosslinking data. Calcium removal prevents S100A4 binding to GM-CSF, whereas monomerization of S100A4/A6/P proteins disrupts S100A4/A6 interaction with GM-CSF and induces a slight decrease in S100P affinity for GM-CSF. Structural modelling indicates the presence in the GM-CSF molecule of a conserved S100A4/A6/P-binding site, consisting of the residues from its termini, helices I and III, some of which are involved in the interaction with GM-CSF receptors. The predicted involvement of the ‘hinge’ region and F89 residue of S100P in GM-CSF recognition was confirmed by mutagenesis. Examination of S100A4/A6/P ability to affect GM-CSF signaling showed that S100A4/A6 inhibit GM-CSF-induced suppression of viability of monocytic THP-1 cells. The ability of the S100 proteins to modulate GM-CSF activity is relevant to progression of various neoplasms and other diseases, according to bioinformatics analysis. The direct regulation of GM-CSF signaling by extracellular forms of the S100 proteins should be taken into account in the clinical use of GM-CSF and development of the therapeutic interventions targeting GM-CSF or its receptors.
中文翻译:

特定 S100 蛋白对粒细胞-巨噬细胞集落刺激因子的识别
粒细胞巨噬细胞集落刺激因子 (GM-CSF) 是一种多效性骨髓生成生长因子和促炎细胞因子,临床上用于多种适应症,是治疗许多疾病的有希望的靶标,包括癌症、多发性硬化症、类风湿性关节炎、牛皮癣、哮喘、COVID-19。我们之前已经证明,多功能 S100 蛋白家族成员的 S100A6 和 S100P 蛋白的二聚 Ca 结合形式对 GM-CSF 具有特异性。为了探测这些相互作用的选择性,通过表面等离子共振光谱研究了重组人 GM-CSF 与 18 种重组人 S100 蛋白的二聚 Ca 负载形式的亲和力。其中,只有 S100A4 蛋白与 GM-CSF 特异性结合,平衡解离常数 为 0.3-2 μM,经内在荧光和化学交联数据证实。钙去除会阻止 S100A4 与 GM-CSF 结合,而 S100A4/A6/P 蛋白的单体化会破坏 S100A4/A6 与 GM-CSF 的相互作用,并导致 S100P 对 GM-CSF 的亲和力略有下降。结构模型表明 GM-CSF 分子中存在保守的 S100A4/A6/P 结合位点,由其末端、螺旋 I 和 III 的残基组成,其中一些残基参与与 GM-CSF 受体的相互作用。通过诱变证实了 S100P 的“铰链”区和 F89 残基参与 GM-CSF 识别的预测。对 S100A4/A6/P 影响 GM-CSF 信号转导的能力的检查表明,S100A4/A6 抑制 GM-CSF 诱导的单核 THP-1 细胞活力的抑制。根据生物信息学分析,S100 蛋白调节 GM-CSF 活性的能力与各种肿瘤和其他疾病的进展相关。在 GM-CSF 的临床使用和针对 GM-CSF 或其受体的治疗干预措施的开发中,应考虑细胞外形式的 S100 蛋白对 GM-CSF 信号传导的直接调节。
更新日期:2024-03-05
中文翻译:

特定 S100 蛋白对粒细胞-巨噬细胞集落刺激因子的识别
粒细胞巨噬细胞集落刺激因子 (GM-CSF) 是一种多效性骨髓生成生长因子和促炎细胞因子,临床上用于多种适应症,是治疗许多疾病的有希望的靶标,包括癌症、多发性硬化症、类风湿性关节炎、牛皮癣、哮喘、COVID-19。我们之前已经证明,多功能 S100 蛋白家族成员的 S100A6 和 S100P 蛋白的二聚 Ca 结合形式对 GM-CSF 具有特异性。为了探测这些相互作用的选择性,通过表面等离子共振光谱研究了重组人 GM-CSF 与 18 种重组人 S100 蛋白的二聚 Ca 负载形式的亲和力。其中,只有 S100A4 蛋白与 GM-CSF 特异性结合,平衡解离常数 为 0.3-2 μM,经内在荧光和化学交联数据证实。钙去除会阻止 S100A4 与 GM-CSF 结合,而 S100A4/A6/P 蛋白的单体化会破坏 S100A4/A6 与 GM-CSF 的相互作用,并导致 S100P 对 GM-CSF 的亲和力略有下降。结构模型表明 GM-CSF 分子中存在保守的 S100A4/A6/P 结合位点,由其末端、螺旋 I 和 III 的残基组成,其中一些残基参与与 GM-CSF 受体的相互作用。通过诱变证实了 S100P 的“铰链”区和 F89 残基参与 GM-CSF 识别的预测。对 S100A4/A6/P 影响 GM-CSF 信号转导的能力的检查表明,S100A4/A6 抑制 GM-CSF 诱导的单核 THP-1 细胞活力的抑制。根据生物信息学分析,S100 蛋白调节 GM-CSF 活性的能力与各种肿瘤和其他疾病的进展相关。在 GM-CSF 的临床使用和针对 GM-CSF 或其受体的治疗干预措施的开发中,应考虑细胞外形式的 S100 蛋白对 GM-CSF 信号传导的直接调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号