当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regio- and Enantioselective Synthesis of Succinimides Bearing All-Carbon Quaternary Centers Using a Chiral Phenanthroline-Palladium Catalyst
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-07 , DOI: 10.1002/adsc.202301486 Yudai Kuroiwa 1 , Masafumi Tamura 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-07 , DOI: 10.1002/adsc.202301486 Yudai Kuroiwa 1 , Masafumi Tamura 1
Affiliation
|
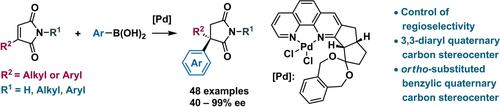
The synthesis of chiral succinimides bearing all‐carbon quaternary stereocenters at the C3 position is important, but remains challenging. The present work demonstrates conjugate additions with catalysis by a chiral 1,10‐phenanthroline‐Pd complex (<b>L1‐</b><b>PdCl</b><sub>2</sub>) that exhibit complete regioselectivity with a high degree of enantioselectivity. These reactions afford chiral 3,3‐disubstituted succinimides having all‐carbon quaternary stereocenters with 40%−99% ee. Importantly, chiral 3,3‐diaryl succinimides could also be obtained with 35%−98% and 40%−99% ee. Moreover, the present <b>L1‐PdCl</b><sub>2</sub>‐catalyzed asymmetric conjugate addition could be performed on the gram scale and was also used to introduce such stereocenters into bioactive molecules.
中文翻译:

使用手性菲咯啉-钯催化剂区域和对映选择性合成带有全碳四元中心的琥珀酰亚胺
在 C3 位具有全碳四元立构中心的手性琥珀酰亚胺的合成很重要,但仍然具有挑战性。目前的工作展示了手性 1,10-菲咯啉-Pd 络合物 (<b>L1-</b><b>PdCl</b><sub>2</sub>) 催化下的共轭加成,表现出完全的区域选择性具有高度的对映选择性。这些反应提供了具有 40%−99% ee 的全碳季立体中心的手性 3,3-二取代琥珀酰亚胺。重要的是,还可以获得 35%−98% 和 40%−99% ee 的手性 3,3-二芳基琥珀酰亚胺。此外,目前的<b>L1-PdCl</b><sub>2</sub>催化的不对称共轭加成可以在克级上进行,并且也可用于将此类立体中心引入生物活性分子。
更新日期:2024-03-07
中文翻译:

使用手性菲咯啉-钯催化剂区域和对映选择性合成带有全碳四元中心的琥珀酰亚胺
在 C3 位具有全碳四元立构中心的手性琥珀酰亚胺的合成很重要,但仍然具有挑战性。目前的工作展示了手性 1,10-菲咯啉-Pd 络合物 (<b>L1-</b><b>PdCl</b><sub>2</sub>) 催化下的共轭加成,表现出完全的区域选择性具有高度的对映选择性。这些反应提供了具有 40%−99% ee 的全碳季立体中心的手性 3,3-二取代琥珀酰亚胺。重要的是,还可以获得 35%−98% 和 40%−99% ee 的手性 3,3-二芳基琥珀酰亚胺。此外,目前的<b>L1-PdCl</b><sub>2</sub>催化的不对称共轭加成可以在克级上进行,并且也可用于将此类立体中心引入生物活性分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号