当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible-light-induced C–S bond formation in the synthesis of 2,4-disubstituted thiazoles through cascade difunctionalization of acetophenone: a greener approach
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2024-03-07 , DOI: 10.1039/d4ob00096j Khushbu Rajput 1 , Vishal Singh 1 , Priya Mahaur 1 , Sundaram Singh 1 , Vandana Srivastava 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2024-03-07 , DOI: 10.1039/d4ob00096j Khushbu Rajput 1 , Vishal Singh 1 , Priya Mahaur 1 , Sundaram Singh 1 , Vandana Srivastava 1
Affiliation
|
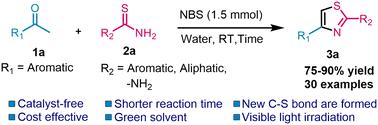
A groundbreaking approach has been developed for synthesizing 2,4-disubstituted thiazoles using an eco-friendly and metal-free approach. This novel method utilizes methyl aryl ketones, N-bromo-succinimide (NBS), and thioamides in water as a green reaction medium under visible light irradiation. Using NBS as a bromine source, the reaction takes place through an in situ α-bromination method. This approach does not require any catalyst, which makes it exceptionally beneficial for the environment. The advantages of this efficient approach are manifold and include the use of greener conditions, absence of metals, easy isolation of products, cost-effectiveness, non-toxicity, and reliance on renewable energy sources like visible light. Moreover, this technique offers higher product purity and excellent yield, enhancing itsappeal.
中文翻译:

通过苯乙酮级联双官能化合成 2,4-二取代噻唑时可见光诱导 C-S 键形成:一种更环保的方法
开发出一种采用环保且无金属的方法合成 2,4-二取代噻唑的突破性方法。这种新方法利用水中的甲基芳基酮、N-溴琥珀酰亚胺(NBS)和硫代酰胺作为可见光照射下的绿色反应介质。使用NBS作为溴源,通过原位α-溴化方法发生反应。这种方法不需要任何催化剂,这使得它对环境特别有益。这种高效方法的优点是多方面的,包括使用更环保的条件、不含金属、易于分离产品、成本效益、无毒以及对可见光等可再生能源的依赖。此外,该技术提供了更高的产品纯度和优异的收率,增强了其吸引力。
更新日期:2024-03-07
中文翻译:

通过苯乙酮级联双官能化合成 2,4-二取代噻唑时可见光诱导 C-S 键形成:一种更环保的方法
开发出一种采用环保且无金属的方法合成 2,4-二取代噻唑的突破性方法。这种新方法利用水中的甲基芳基酮、N-溴琥珀酰亚胺(NBS)和硫代酰胺作为可见光照射下的绿色反应介质。使用NBS作为溴源,通过原位α-溴化方法发生反应。这种方法不需要任何催化剂,这使得它对环境特别有益。这种高效方法的优点是多方面的,包括使用更环保的条件、不含金属、易于分离产品、成本效益、无毒以及对可见光等可再生能源的依赖。此外,该技术提供了更高的产品纯度和优异的收率,增强了其吸引力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号