当前位置:
X-MOL 学术
›
Clin. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Safety and Efficacy of Efruxifermin in Combination With a GLP-1 Receptor Agonist in Patients With NASH/MASH and Type 2 Diabetes in a Randomized Phase 2 Study
Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2024-03-04 , DOI: 10.1016/j.cgh.2024.02.022 Stephen A. Harrison , Juan P. Frias , K. Jean Lucas , Gary Reiss , Guy Neff , Sureka Bollepalli , Yan Su , Doreen Chan , Erik J. Tillman , Ali Moulton , Brittany de Temple , Arian Zari , Reshma Shringarpure , Timothy Rolph , Andrew Cheng , Kitty Yale
Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2024-03-04 , DOI: 10.1016/j.cgh.2024.02.022 Stephen A. Harrison , Juan P. Frias , K. Jean Lucas , Gary Reiss , Guy Neff , Sureka Bollepalli , Yan Su , Doreen Chan , Erik J. Tillman , Ali Moulton , Brittany de Temple , Arian Zari , Reshma Shringarpure , Timothy Rolph , Andrew Cheng , Kitty Yale
|
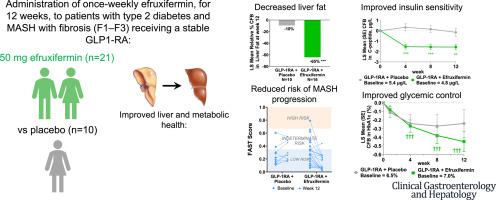
In phase 2 studies, efruxifermin, an Fc–FGF21 analog, significantly reduced steatohepatitis and fibrosis in patients with non-alcoholic steatohepatitis, now called metabolic dysfunction-associated steatohepatitis (MASH), for which there is no approved treatment. Type 2 diabetes (T2D) and obesity are prevalent among patients with MASH and increasingly treated with glucagon-like peptide-1 receptor agonists (GLP-1RAs). This study evaluated the safety and efficacy of efruxifermin in patients with MASH, fibrosis, and T2D taking a GLP-1RA. Cohort D was a double-blind, placebo-controlled, phase 2b study in adults with T2D and MASH with fibrosis (F1–F3) on stable GLP-1RA therapy randomized (2:1) to receive efruxifermin 50 mg or placebo, once weekly for 12 weeks. The primary endpoint was safety and tolerability of efruxifermin added to a stable dose of GLP-1RA. Secondary endpoints included changes in hepatic fat fraction (HFF), markers of liver injury and fibrosis, and metabolic parameters. Adults (N = 31) with T2D and MASH fibrosis (F1–F3) on a stable GLP-1RA (semaglutide, 48.4%; dulaglutide, 45.2%; liraglutide, 6.5%) received efruxifermin 50 mg (n = 21) or placebo (n = 10) for 12 weeks. The addition of efruxifermin to a GLP-1RA appeared safe and well-tolerated. The most frequent efruxifermin-related adverse events were mild to moderate gastrointestinal events. One patient receiving efruxifermin discontinued due to nausea, and another withdrew consent. There were no treatment-related serious adverse events. After 12 weeks, efruxifermin reduced HFF by 65% ( < .0001 vs placebo) compared with a 10% reduction for placebo (GLP-1RA alone). Efruxifermin also improved noninvasive markers of liver injury, fibrosis, glucose, and lipid metabolism while maintaining GLP-1RA–mediated weight loss. The tolerability profile of efruxifermin added to GLP-1RA appeared comparable to that of either drug alone, while also significantly reducing HFF and noninvasive markers of fibrosis in patients with MASH and T2D. Liver health in patients already on a GLP-1RA may be further improved by addition of efruxifermin. , Number: .
中文翻译:

在一项随机 2 期研究中,Efruxifermin 与 GLP-1 受体激动剂联合治疗 NASH/MASH 和 2 型糖尿病患者的安全性和有效性
在 2 期研究中,efruxifermin(一种 Fc-FGF21 类似物)可显着减少非酒精性脂肪性肝炎(现在称为代谢功能障碍相关脂肪性肝炎 (MASH))患者的脂肪性肝炎和纤维化,目前尚无批准的治疗方法。 2 型糖尿病 (T2D) 和肥胖症在 MASH 患者中普遍存在,并且越来越多地接受胰高血糖素样肽 1 受体激动剂 (GLP-1RA) 治疗。本研究评估了 efruxifermin 对于服用 GLP-1RA 的 MASH、纤维化和 T2D 患者的安全性和有效性。 D 组是一项双盲、安慰剂对照、2b 期研究,受试者为接受稳定 GLP-1RA 治疗的 T2D 和 MASH 伴纤维化 (F1–F3) 成人患者,随机 (2:1) 接受 efruxifermin 50 mg 或安慰剂,每周一次12 周。主要终点是添加到稳定剂量的 GLP-1RA 中的 efruxifermin 的安全性和耐受性。次要终点包括肝脂肪分数(HFF)、肝损伤和纤维化标志物以及代谢参数的变化。使用稳定 GLP-1RA(索马鲁肽,48.4%;度拉鲁肽,45.2%;利拉鲁肽,6.5%)患有 T2D 和 MASH 纤维化 (F1–F3) 的成人 (N = 31) 接受 efruxifermin 50 mg (n = 21) 或安慰剂( n = 10) 12 周。在 GLP-1RA 中添加 efruxifermin 似乎是安全且耐受性良好的。最常见的与 efruxifermin 相关的不良事件是轻度至中度胃肠道事件。一名接受 efruxifermin 治疗的患者因恶心而停药,另一名患者撤回同意。没有出现与治疗相关的严重不良事件。 12 周后,efruxifermin 使 HFF 降低 65%(与安慰剂相比 < 0.0001),而安慰剂(单独使用 GLP-1RA)则降低 10%。 Efruxifermin 还改善了肝损伤、纤维化、葡萄糖和脂质代谢的非侵入性标志物,同时维持 GLP-1RA 介导的体重减轻。添加到 GLP-1RA 中的 efruxifermin 的耐受性似乎与单独使用任何一种药物相当,同时还显着降低了 MASH 和 T2D 患者的 HFF 和非侵入性纤维化标志物。已经接受 GLP-1RA 治疗的患者的肝脏健康可能会通过添加 efruxifermin 得到进一步改善。 , 数字: 。
更新日期:2024-03-04
中文翻译:

在一项随机 2 期研究中,Efruxifermin 与 GLP-1 受体激动剂联合治疗 NASH/MASH 和 2 型糖尿病患者的安全性和有效性
在 2 期研究中,efruxifermin(一种 Fc-FGF21 类似物)可显着减少非酒精性脂肪性肝炎(现在称为代谢功能障碍相关脂肪性肝炎 (MASH))患者的脂肪性肝炎和纤维化,目前尚无批准的治疗方法。 2 型糖尿病 (T2D) 和肥胖症在 MASH 患者中普遍存在,并且越来越多地接受胰高血糖素样肽 1 受体激动剂 (GLP-1RA) 治疗。本研究评估了 efruxifermin 对于服用 GLP-1RA 的 MASH、纤维化和 T2D 患者的安全性和有效性。 D 组是一项双盲、安慰剂对照、2b 期研究,受试者为接受稳定 GLP-1RA 治疗的 T2D 和 MASH 伴纤维化 (F1–F3) 成人患者,随机 (2:1) 接受 efruxifermin 50 mg 或安慰剂,每周一次12 周。主要终点是添加到稳定剂量的 GLP-1RA 中的 efruxifermin 的安全性和耐受性。次要终点包括肝脂肪分数(HFF)、肝损伤和纤维化标志物以及代谢参数的变化。使用稳定 GLP-1RA(索马鲁肽,48.4%;度拉鲁肽,45.2%;利拉鲁肽,6.5%)患有 T2D 和 MASH 纤维化 (F1–F3) 的成人 (N = 31) 接受 efruxifermin 50 mg (n = 21) 或安慰剂( n = 10) 12 周。在 GLP-1RA 中添加 efruxifermin 似乎是安全且耐受性良好的。最常见的与 efruxifermin 相关的不良事件是轻度至中度胃肠道事件。一名接受 efruxifermin 治疗的患者因恶心而停药,另一名患者撤回同意。没有出现与治疗相关的严重不良事件。 12 周后,efruxifermin 使 HFF 降低 65%(与安慰剂相比 < 0.0001),而安慰剂(单独使用 GLP-1RA)则降低 10%。 Efruxifermin 还改善了肝损伤、纤维化、葡萄糖和脂质代谢的非侵入性标志物,同时维持 GLP-1RA 介导的体重减轻。添加到 GLP-1RA 中的 efruxifermin 的耐受性似乎与单独使用任何一种药物相当,同时还显着降低了 MASH 和 T2D 患者的 HFF 和非侵入性纤维化标志物。已经接受 GLP-1RA 治疗的患者的肝脏健康可能会通过添加 efruxifermin 得到进一步改善。 , 数字: 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号