当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploitation of Mechanistic Product Selectivity for the Two-Step Synthesis of Optically Active Bio-Derived Cyclic Carbonates Incorporating Amino Acids
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-03-06 , DOI: 10.1002/ejoc.202400219 Diego Jaraba Cabrera 1 , Lucía Álvarez-Miguel 1 , Adrián Hernando Rodríguez 1 , Alex Hamilton 2 , Marta E. G. Mosquera 1 , Christopher J. Whiteoak 3
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-03-06 , DOI: 10.1002/ejoc.202400219 Diego Jaraba Cabrera 1 , Lucía Álvarez-Miguel 1 , Adrián Hernando Rodríguez 1 , Alex Hamilton 2 , Marta E. G. Mosquera 1 , Christopher J. Whiteoak 3
Affiliation
|
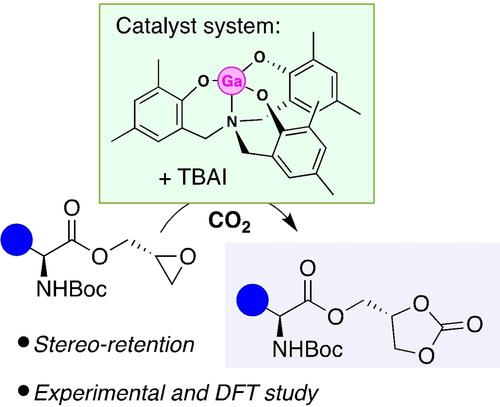
Synthesis of cyclic carbonates bearing amino acid functionality is described. The use of enantiopure amino acids and glycidol, in the initial formation of the substrate, combined with the stereo-retentive mechanism of the cycloaddition with CO2 furnishes bio-derived cyclic carbonates which display a range of optical activities. A DFT study provides important insights into the operative mechanism.
中文翻译:

利用机械产物选择性两步合成含有氨基酸的光学活性生物衍生环状碳酸酯
描述了带有氨基酸官能团的环状碳酸酯的合成。在底物的初始形成中使用对映体纯氨基酸和缩水甘油,结合与CO 2环加成的立体保留机制,提供了显示出一系列光学活性的生物来源的环状碳酸酯。 DFT 研究提供了对其运行机制的重要见解。
更新日期:2024-03-06
中文翻译:

利用机械产物选择性两步合成含有氨基酸的光学活性生物衍生环状碳酸酯
描述了带有氨基酸官能团的环状碳酸酯的合成。在底物的初始形成中使用对映体纯氨基酸和缩水甘油,结合与CO 2环加成的立体保留机制,提供了显示出一系列光学活性的生物来源的环状碳酸酯。 DFT 研究提供了对其运行机制的重要见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号