当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergy of nanocrystalline carbon nitride with Cu single atom catalyst leads to selective photocatalytic reduction of CO2 to methanol
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2024-03-06 , DOI: 10.1039/d4se00028e Tara M. LeMercier 1 , Madasamy Thangamuthu 1 , Emerson C. Kohlrausch 1 , Yifan Chen 1 , Craig T. Stoppiello 2 , Michael W. Fay 3 , Graham A. Rance 3 , Gazi N. Aliev 4 , Wolfgang Theis 4 , Johannes Biskupek 5 , Ute Kaiser 5 , Anabel E. Lanterna 1 , Jesum Alves Fernandes 1 , Andrei N. Khlobystov 1
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2024-03-06 , DOI: 10.1039/d4se00028e Tara M. LeMercier 1 , Madasamy Thangamuthu 1 , Emerson C. Kohlrausch 1 , Yifan Chen 1 , Craig T. Stoppiello 2 , Michael W. Fay 3 , Graham A. Rance 3 , Gazi N. Aliev 4 , Wolfgang Theis 4 , Johannes Biskupek 5 , Ute Kaiser 5 , Anabel E. Lanterna 1 , Jesum Alves Fernandes 1 , Andrei N. Khlobystov 1
Affiliation
|
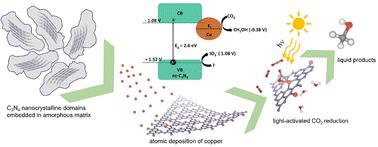
Carbon nitride (C3N4) possesses both a band gap in the visible range and a low-lying conduction band potential, suitable for water splitting and CO2 reduction reactions (CO2RR). Yet, bulk C3N4 (b-C3N4) suffers from structural disorder leading to sluggish reaction kinetics. This can be improved by graphitisation; however, current processes in the literature, lead to a variety of graphitised C3N4 (g-C3N4), making it difficult to link the degrees of graphitisation with the functional properties. Herein, we employ complementary analyses, including electrochemical impedance, photoluminescence, and photocurrent, to elucidate structure–property–function relationships. Guided by the descriptors, we developed a facile two-step annealing method that yields nanocrystalline carbon nitride (nc-C3N4), comprising nanoscale graphitic domains within an amorphous matrix. The nanocrystalline grains of nc-C3N4 allow effective immobilisation of Cu atoms and stabilisation of low oxidation states (Cu(I)). Electron microscopy and energy-dispersive X-ray spectroscopy demonstrate that Cu is atomically dispersed. Importantly, the addition of only 0.11 wt% of copper to nc-C3N4 drastically decreases the charge recombination and resistance to change transfer. The synergy of the Cu single-atom catalyst and nanocrystalline domains in carbon nitride (Cu/nc-C3N4) leads to a remarkable 99% selectivity towards methanol production with a rate of 316 μmol gcat−1 h−1 during the photocatalytic CO2RR, which is absent in Cu/b-C3N4.
中文翻译:

纳米晶氮化碳与铜单原子催化剂的协同作用导致二氧化碳选择性光催化还原为甲醇
氮化碳(C 3 N 4 )同时具有可见光范围内的带隙和低导带电势,适合水分解和CO 2还原反应(CO 2 RR)。然而,本体C 3 N 4 (bC 3 N 4 ) 存在结构紊乱,导致反应动力学缓慢。这可以通过石墨化来改善;然而,目前文献中的工艺导致产生多种石墨化C 3 N 4 (gC 3 N 4 ),使得难以将石墨化程度与功能特性联系起来。在这里,我们采用补充分析,包括电化学阻抗、光致发光和光电流,来阐明结构-性能-功能关系。在描述符的指导下,我们开发了一种简便的两步退火方法,可产生纳米晶体氮化碳(nc-C 3 N 4),其中包含非晶基质内的纳米级石墨域。 nc-C 3 N 4的纳米晶粒可以有效固定铜原子并稳定低氧化态(Cu(I))。电子显微镜和能量色散 X 射线光谱表明 Cu 是原子分散的。重要的是,在 nc-C 3 N 4中仅添加 0.11 wt% 的铜就大大降低了电荷复合和电荷转移阻力。 Cu单原子催化剂和氮化碳(Cu/nc-C 3 N 4 )中的纳米晶域的协同作用使得甲醇生产选择性显着达到99%,在反应过程中,甲醇生成率为316 μmol g cat -1 h -1光催化CO 2 RR,Cu/bC 3 N 4中不存在。
更新日期:2024-03-06
中文翻译:

纳米晶氮化碳与铜单原子催化剂的协同作用导致二氧化碳选择性光催化还原为甲醇
氮化碳(C 3 N 4 )同时具有可见光范围内的带隙和低导带电势,适合水分解和CO 2还原反应(CO 2 RR)。然而,本体C 3 N 4 (bC 3 N 4 ) 存在结构紊乱,导致反应动力学缓慢。这可以通过石墨化来改善;然而,目前文献中的工艺导致产生多种石墨化C 3 N 4 (gC 3 N 4 ),使得难以将石墨化程度与功能特性联系起来。在这里,我们采用补充分析,包括电化学阻抗、光致发光和光电流,来阐明结构-性能-功能关系。在描述符的指导下,我们开发了一种简便的两步退火方法,可产生纳米晶体氮化碳(nc-C 3 N 4),其中包含非晶基质内的纳米级石墨域。 nc-C 3 N 4的纳米晶粒可以有效固定铜原子并稳定低氧化态(Cu(I))。电子显微镜和能量色散 X 射线光谱表明 Cu 是原子分散的。重要的是,在 nc-C 3 N 4中仅添加 0.11 wt% 的铜就大大降低了电荷复合和电荷转移阻力。 Cu单原子催化剂和氮化碳(Cu/nc-C 3 N 4 )中的纳米晶域的协同作用使得甲醇生产选择性显着达到99%,在反应过程中,甲醇生成率为316 μmol g cat -1 h -1光催化CO 2 RR,Cu/bC 3 N 4中不存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号