Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Air–Liquid Interface Microfluidic Monitoring Sensor Platform for Studying Autophagy Regulation after PM2.5 Exposure
ACS Sensors ( IF 8.9 ) Pub Date : 2024-03-04 , DOI: 10.1021/acssensors.3c01744 Lulu Zheng 1 , Zhijin Yang 1 , Zhiwei Xue 1 , Mengya Chen 1 , Yule Zhang 1 , Shuqi Cai 1 , Kejie Zheng 1 , Bo Dai 1 , Sixiu Liu 2 , Songlin Zhuang 1, 3 , Guodong Sui 2 , Dawei Zhang 1, 3, 4
ACS Sensors ( IF 8.9 ) Pub Date : 2024-03-04 , DOI: 10.1021/acssensors.3c01744 Lulu Zheng 1 , Zhijin Yang 1 , Zhiwei Xue 1 , Mengya Chen 1 , Yule Zhang 1 , Shuqi Cai 1 , Kejie Zheng 1 , Bo Dai 1 , Sixiu Liu 2 , Songlin Zhuang 1, 3 , Guodong Sui 2 , Dawei Zhang 1, 3, 4
Affiliation
|
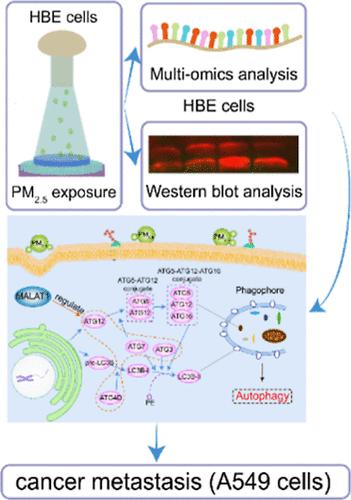
Undoubtedly, a deep understanding of PM2.5-induced tumor metastasis at the molecular level can contribute to improving the therapeutic effects of related diseases. However, the underlying molecular mechanism of fine particle exposure through long noncoding RNA (lncRNA) regulation in autophagy and, ultimately, lung cancer (LC) metastasis remains elusive; on the other hand, the related monitoring sensor platform used to investigate autophagy and cell migration is lacking. Herein, this study performed an air–liquid interface microfluidic monitoring sensor (AIMMS) platform to analyze human bronchial epithelial cells after PM2.5 stimulation. The multiomics analysis [RNA sequencing (RNA-seq) on lncRNA and mRNA expressions separately] showed that MALAT1 was highly expressed in the PM2.5 treatment group. Furthermore, RNA-seq analysis demonstrated that autophagy-related pathways were activated. Notably, the main mRNAs associated with autophagy regulation, including ATG4D, ATG12, ATG7, and ATG3, were upregulated. Inhibition or downregulation of MALAT1 inhibited autophagy via the ATG4D/ATG12/ATG7/ATG3 pathway after PM2.5 exposure and ultimately suppressed LC metastasis. Thus, based on the AIMMS platform, we found that MALAT1 might become a promising therapeutic target. Furthermore, this low-cost AIMMS system as a fluorescence sensor integrated with the cell-monitor module could be employed to study LC migration after PM2.5 exposure. With the fluorescence cell-monitoring module, the platform could be used to observe the migration of LC cells and construct the tumor metastasis model. In the future, several fluorescence probes, including nanoprobes, could be used in the AIMMS platform to investigate many other biological processes, especially cell interaction and migration, in the fields of toxicology and pharmacology.
中文翻译:

用于研究 PM2.5 暴露后自噬调节的气液界面微流控传感器平台
无疑,从分子水平深入了解PM 2.5引起的肿瘤转移有助于提高相关疾病的治疗效果。然而,细颗粒暴露通过长非编码RNA (lncRNA) 调节自噬以及最终肺癌 (LC) 转移的潜在分子机制仍然难以捉摸。另一方面,缺乏用于研究自噬和细胞迁移的相关监测传感器平台。在此,本研究使用气液界面微流体监测传感器(AIMMS)平台来分析 PM 2.5刺激后的人支气管上皮细胞。多组学分析[分别对lncRNA和mRNA表达进行RNA测序(RNA-seq)]显示MALAT1在PM 2.5治疗组中高表达。此外,RNA-seq 分析表明自噬相关途径被激活。值得注意的是,与自噬调节相关的主要 mRNA,包括 ATG4D、ATG12、ATG7 和 ATG3,均上调。 PM 2.5暴露后,抑制或下调 MALAT1 可通过 ATG4D/ATG12/ATG7/ATG3 途径抑制自噬,最终抑制 LC 转移。因此,基于AIMMS平台,我们发现MALAT1可能成为一个有前途的治疗靶点。此外,这种低成本 AIMMS 系统作为与细胞监测模块集成的荧光传感器,可用于研究 PM 2.5暴露后的 LC 迁移。配合荧光细胞监测模块,该平台可用于观察LC细胞的迁移,构建肿瘤转移模型。未来,包括纳米探针在内的多种荧光探针可用于AIMMS平台,以研究毒理学和药理学领域的许多其他生物过程,特别是细胞相互作用和迁移。
更新日期:2024-03-04
中文翻译:

用于研究 PM2.5 暴露后自噬调节的气液界面微流控传感器平台
无疑,从分子水平深入了解PM 2.5引起的肿瘤转移有助于提高相关疾病的治疗效果。然而,细颗粒暴露通过长非编码RNA (lncRNA) 调节自噬以及最终肺癌 (LC) 转移的潜在分子机制仍然难以捉摸。另一方面,缺乏用于研究自噬和细胞迁移的相关监测传感器平台。在此,本研究使用气液界面微流体监测传感器(AIMMS)平台来分析 PM 2.5刺激后的人支气管上皮细胞。多组学分析[分别对lncRNA和mRNA表达进行RNA测序(RNA-seq)]显示MALAT1在PM 2.5治疗组中高表达。此外,RNA-seq 分析表明自噬相关途径被激活。值得注意的是,与自噬调节相关的主要 mRNA,包括 ATG4D、ATG12、ATG7 和 ATG3,均上调。 PM 2.5暴露后,抑制或下调 MALAT1 可通过 ATG4D/ATG12/ATG7/ATG3 途径抑制自噬,最终抑制 LC 转移。因此,基于AIMMS平台,我们发现MALAT1可能成为一个有前途的治疗靶点。此外,这种低成本 AIMMS 系统作为与细胞监测模块集成的荧光传感器,可用于研究 PM 2.5暴露后的 LC 迁移。配合荧光细胞监测模块,该平台可用于观察LC细胞的迁移,构建肿瘤转移模型。未来,包括纳米探针在内的多种荧光探针可用于AIMMS平台,以研究毒理学和药理学领域的许多其他生物过程,特别是细胞相互作用和迁移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号