当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical oxidative dehydrogenation aromatization of cyclohex-2-enone and amines to 1,4-phenylenediamine
Green Chemistry ( IF 9.8 ) Pub Date : 2024-03-04 , DOI: 10.1039/d3gc04869a Jiayu Hu 1 , Rui Ma 1 , Jingcheng Hu 1 , Xing Liu 1 , Xue Liu 1 , Haoyu He 1 , Hong Yi 1 , Aiwen Lei 1
Green Chemistry ( IF 9.8 ) Pub Date : 2024-03-04 , DOI: 10.1039/d3gc04869a Jiayu Hu 1 , Rui Ma 1 , Jingcheng Hu 1 , Xing Liu 1 , Xue Liu 1 , Haoyu He 1 , Hong Yi 1 , Aiwen Lei 1
Affiliation
|
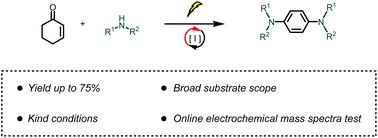
Aromatic amines, crucial organic entities, exist in drugs, natural products, organic materials, and catalysts. The introduction of amines to aromatics without using metal catalysts and chemical oxidants poses a formidable challenge. Recent years have witnessed extensive developments in the oxidative aromatization of aniline. In this context, we present the electrochemical cross-dehydrogenation aromatization (ECDA) reaction involving cyclohex-2-enone and amines, culminating in the synthesis of 1,4-phenylenediamine, devoid of supplementary metal catalysts and chemical oxidants. The broad applicability of the reaction substrate is underscored, demonstrating its compatibility with select natural and pharmaceutical molecules, thus showing considerable potential for the synthesis of bioactive compounds. The mechanistic underpinnings of the reaction were probed using online electrochemical mass spectrometry.
中文翻译:

环己烯酮和胺电化学氧化脱氢芳构化制1,4-苯二胺
芳香胺是重要的有机实体,存在于药物、天然产物、有机材料和催化剂中。在不使用金属催化剂和化学氧化剂的情况下将胺引入芳烃中提出了巨大的挑战。近年来,苯胺的氧化芳构化得到了广泛的发展。在此背景下,我们提出了涉及环己-2-烯酮和胺的电化学交叉脱氢芳构化(ECDA)反应,最终合成1,4-苯二胺,无需补充金属催化剂和化学氧化剂。强调了反应底物的广泛适用性,证明了其与精选的天然和药物分子的相容性,从而显示出合成生物活性化合物的巨大潜力。使用在线电化学质谱法探讨了该反应的机理基础。
更新日期:2024-03-04
中文翻译:

环己烯酮和胺电化学氧化脱氢芳构化制1,4-苯二胺
芳香胺是重要的有机实体,存在于药物、天然产物、有机材料和催化剂中。在不使用金属催化剂和化学氧化剂的情况下将胺引入芳烃中提出了巨大的挑战。近年来,苯胺的氧化芳构化得到了广泛的发展。在此背景下,我们提出了涉及环己-2-烯酮和胺的电化学交叉脱氢芳构化(ECDA)反应,最终合成1,4-苯二胺,无需补充金属催化剂和化学氧化剂。强调了反应底物的广泛适用性,证明了其与精选的天然和药物分子的相容性,从而显示出合成生物活性化合物的巨大潜力。使用在线电化学质谱法探讨了该反应的机理基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号