当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cation Effects on the Acidic Oxygen Reduction Reaction at Carbon Surfaces
ACS Energy Letters ( IF 22.0 ) Pub Date : 2024-03-01 , DOI: 10.1021/acsenergylett.3c02743 J. L. Hübner 1 , L. E. B. Lucchetti 2 , H. N. Nong 1 , D. I. Sharapa 3 , B. Paul 1 , M. Kroschel 1 , J. Kang 1 , D. Teschner 4, 5 , S. Behrens 3 , F. Studt 3 , A. Knop-Gericke 4, 5 , S. Siahrostami 6 , P. Strasser 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2024-03-01 , DOI: 10.1021/acsenergylett.3c02743 J. L. Hübner 1 , L. E. B. Lucchetti 2 , H. N. Nong 1 , D. I. Sharapa 3 , B. Paul 1 , M. Kroschel 1 , J. Kang 1 , D. Teschner 4, 5 , S. Behrens 3 , F. Studt 3 , A. Knop-Gericke 4, 5 , S. Siahrostami 6 , P. Strasser 1
Affiliation
|
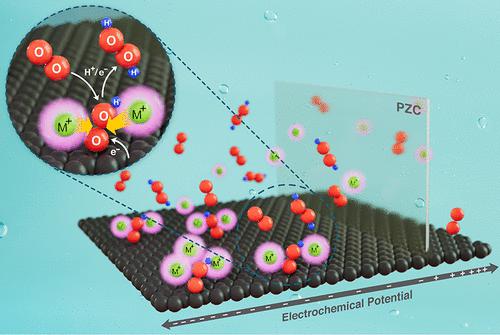
Hydrogen peroxide (H2O2) is a widely used green oxidant. Until now, research has focused on the development of efficient catalysts for the two-electron oxygen reduction reaction (2e– ORR). However, electrolyte effects on the 2e– ORR have remained little understood. We report a significant effect of alkali metal cations (AMCs) on carbons in acidic environments. The presence of AMCs at a glassy carbon electrode shifts the half wave potential from −0.48 to −0.22 VRHE. This cation-induced enhancement effect exhibits a uniquely sensitive on/off switching behavior depending on the voltammetric protocol. Voltammetric and in situ X-ray photoemission spectroscopic evidence is presented, supporting a controlling role of the potential of zero charge of the catalytic enhancement. Density functional theory calculations associate the enhancement with stabilization of the *OOH key intermediate as a result of locally induced field effects from the AMCs. Finally, we developed a refined reaction mechanism for the H2O2 production in the presence of AMCs.
中文翻译:

阳离子对碳表面酸性氧还原反应的影响
过氧化氢(H 2 O 2)是一种广泛使用的绿色氧化剂。到目前为止,研究重点是开发用于双电子氧还原反应 (2e – ORR) 的高效催化剂。然而,电解质对 2e – ORR 的影响仍知之甚少。我们报告了碱金属阳离子(AMC)在酸性环境中对碳的显着影响。玻碳电极上 AMC 的存在将半波电位从 -0.48 V RHE 移至 -0.22 V RHE。这种阳离子诱导的增强效应表现出独特的敏感开/关切换行为,具体取决于伏安法协议。伏安法和原位X 射线光电子能谱证据显示,支持催化增强的零电荷电势的控制作用。密度泛函理论计算将*OOH 关键中间体的增强与稳定性联系起来,这是 AMC 局部感应场效应的结果。最后,我们开发了一种在 AMC 存在下生产H 2 O 2的精细反应机制。
更新日期:2024-03-01
中文翻译:

阳离子对碳表面酸性氧还原反应的影响
过氧化氢(H 2 O 2)是一种广泛使用的绿色氧化剂。到目前为止,研究重点是开发用于双电子氧还原反应 (2e – ORR) 的高效催化剂。然而,电解质对 2e – ORR 的影响仍知之甚少。我们报告了碱金属阳离子(AMC)在酸性环境中对碳的显着影响。玻碳电极上 AMC 的存在将半波电位从 -0.48 V RHE 移至 -0.22 V RHE。这种阳离子诱导的增强效应表现出独特的敏感开/关切换行为,具体取决于伏安法协议。伏安法和原位X 射线光电子能谱证据显示,支持催化增强的零电荷电势的控制作用。密度泛函理论计算将*OOH 关键中间体的增强与稳定性联系起来,这是 AMC 局部感应场效应的结果。最后,我们开发了一种在 AMC 存在下生产H 2 O 2的精细反应机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号