当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal-Structure Control of Molecular Semiconductors by Methylthiolation: Toward Ultrahigh Mobility
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2024-03-01 , DOI: 10.1021/acs.accounts.3c00756 Kazuo Takimiya 1, 2, 3 , Kirill Bulgarevich 2 , Kohsuke Kawabata 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2024-03-01 , DOI: 10.1021/acs.accounts.3c00756 Kazuo Takimiya 1, 2, 3 , Kirill Bulgarevich 2 , Kohsuke Kawabata 1, 2
Affiliation
|
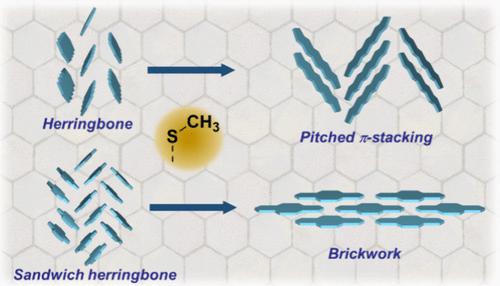
The crystal structure of organic semiconductors has been regarded as one of the crucial factors for realizing high-performance electronic devices, such as organic field-effect transistors. However, although the control of crystal structures of organic semiconductors has been examined in the last two decades of intensive efforts of the development of organic semiconductors, active measures to control crystal structures enabling high carrier mobility are still limited. In 2016, our research group noticed that regioselective methylthiolation could provide a selective crystal structure change from an ordinary herringbone structure to a pitched π-stacking structure, similar to the crystal structure of rubrene, in the benzo[1,2-b:4,5-b′]dithiophene (BDT) system. Following this serendipitous finding, our group systematically investigated the relationship between the molecular and crystal structures of a range of methylthiolated aromatic and heteroaromatic compounds.
中文翻译:

通过甲硫基化控制分子半导体的晶体结构:迈向超高迁移率
有机半导体的晶体结构被认为是实现有机场效应晶体管等高性能电子器件的关键因素之一。然而,尽管在过去二十年有机半导体发展的深入努力中已经对有机半导体晶体结构的控制进行了研究,但控制晶体结构以实现高载流子迁移率的积极措施仍然有限。2016年,我们的研究小组注意到,区域选择性甲基硫基化可以在苯并[1,2- b :4, 5- b ']二噻吩(BDT)系统。根据这一偶然发现,我们的小组系统地研究了一系列甲硫基芳香族和杂芳香族化合物的分子和晶体结构之间的关系。
更新日期:2024-03-01
中文翻译:

通过甲硫基化控制分子半导体的晶体结构:迈向超高迁移率
有机半导体的晶体结构被认为是实现有机场效应晶体管等高性能电子器件的关键因素之一。然而,尽管在过去二十年有机半导体发展的深入努力中已经对有机半导体晶体结构的控制进行了研究,但控制晶体结构以实现高载流子迁移率的积极措施仍然有限。2016年,我们的研究小组注意到,区域选择性甲基硫基化可以在苯并[1,2- b :4, 5- b ']二噻吩(BDT)系统。根据这一偶然发现,我们的小组系统地研究了一系列甲硫基芳香族和杂芳香族化合物的分子和晶体结构之间的关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号