当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of the intramolecular Diels–Alder vinylarene (IMDAV) reaction for the synthesis of benzo-, carbocyclo-, thienothiopheneisoindolecarboxylic acids and its limitations
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2024-03-01 , DOI: 10.1039/d3ob01933k Elizaveta D. Yakovleva 1 , Evgeniya R. Shelukho 1 , Maryana A. Nadirova 1 , Pavel P. Erokhin 1 , Daria N. Simakova 1 , Victor N. Khrustalev 1, 2 , Mikhail S. Grigoriev 3 , Anton P. Novikov 1, 3 , Anna A. Romanycheva 4 , Anton A. Shetnev 5 , Olga P. Bychkova 6 , Alexey S. Trenin 6 , Fedor I. Zubkov 1 , Vladimir P. Zaytsev 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2024-03-01 , DOI: 10.1039/d3ob01933k Elizaveta D. Yakovleva 1 , Evgeniya R. Shelukho 1 , Maryana A. Nadirova 1 , Pavel P. Erokhin 1 , Daria N. Simakova 1 , Victor N. Khrustalev 1, 2 , Mikhail S. Grigoriev 3 , Anton P. Novikov 1, 3 , Anna A. Romanycheva 4 , Anton A. Shetnev 5 , Olga P. Bychkova 6 , Alexey S. Trenin 6 , Fedor I. Zubkov 1 , Vladimir P. Zaytsev 1
Affiliation
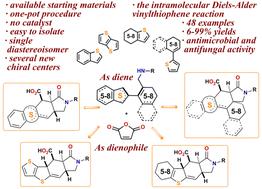
|
Thienylallylamines, readily accessible from the corresponding thienyl aldehydes, react with maleic and trifluoromethylmaleic anhydrides leading to the formation of acids with a thieno[2,3-f]isoindole core. The reaction sequence involves two successive steps: acylation of the nitrogen atom of the initial allylamine and the intramolecular Diels–Alder vinylarene (IMDAV) reaction. The scope and limitations of the proposed method were thoroughly investigated. It has been revealed with the aid of X-ray analysis and DFT calculations that the key step, the IMDAV reaction, proceeds through an exo-transition state, giving rise to the exclusive formation of a single diastereomer of the target heterocycle. The obtained functionally substituted thieno[2,3-f]isoindole carboxylic acids are potentially useful substrates for further transformations and bioscreening. The antimicrobial evaluation of the obtained compounds revealed that 1-oxo-2-(3-(trifluoromethyl)phenyl)hexahydrobenzo[4,5]thieno[2,3-f]isoindole-10-carboxylic acid is the most active sample in the synthesized library. It exhibits antibacterial activity against sensitive strains of Gram-positive bacteria, including S. aureus, Enterococcus faecium, Bacillus cereus, and Micrococcus luteus, as well as the Gram-negative bacteria E. coli and Pseudomonas fluorescens, with MIC values ranging from 4 to 64 μg mL−1. 9-Oxo-8-phenyloctahydronaphtho[2,1-d]thieno[2,3-f]isoindole-10-carboxylic acid showed antifungal activity against yeast culture C. albicans with a MIC value of 32 μM.
中文翻译:

分子内狄尔斯-阿尔德乙烯基芳烃(IMDAV)反应在苯并、碳环、噻吩并噻吩异吲哚甲酸合成中的应用及其局限性
噻吩基烯丙胺很容易从相应的噻吩基醛中获得,与马来酸酐和三氟甲基马来酸酐反应,形成具有噻吩并[2,3- f ]异吲哚核心的酸。该反应序列涉及两个连续步骤:初始烯丙胺的氮原子的酰化和分子内狄尔斯-阿尔德乙烯基芳烃(IMDAV)反应。对所提出方法的范围和局限性进行了彻底研究。借助X射线分析和DFT计算表明,关键步骤IMDAV反应通过外过渡态进行,导致目标杂环的单一非对映异构体的排他性形成。获得的功能取代的噻吩并[2,3- f ]异吲哚羧酸是用于进一步转化和生物筛选的潜在有用底物。对所得化合物的抗菌评价表明,1-氧代-2-(3-(三氟甲基)苯基)六氢苯并[4,5]噻吩并[2,3- f ]异吲哚-10-甲酸是该化合物中活性最高的样品。合成文库。它对革兰氏阳性菌敏感菌株,包括金黄色葡萄球菌、屎肠球菌、蜡样芽孢杆菌和藤黄微球菌,以及革兰氏阴性菌大肠杆菌和荧光假单胞菌具有抗菌活性,MIC值范围为4至64μg·mL -1。9-Oxo-8-苯基八氢萘并[2,1- d ]噻吩并[2,3 - f ]异吲哚-10-羧酸对酵母培养物白色念珠菌具有抗真菌活性,MIC 值为 32 μM。
更新日期:2024-03-01
中文翻译:

分子内狄尔斯-阿尔德乙烯基芳烃(IMDAV)反应在苯并、碳环、噻吩并噻吩异吲哚甲酸合成中的应用及其局限性
噻吩基烯丙胺很容易从相应的噻吩基醛中获得,与马来酸酐和三氟甲基马来酸酐反应,形成具有噻吩并[2,3- f ]异吲哚核心的酸。该反应序列涉及两个连续步骤:初始烯丙胺的氮原子的酰化和分子内狄尔斯-阿尔德乙烯基芳烃(IMDAV)反应。对所提出方法的范围和局限性进行了彻底研究。借助X射线分析和DFT计算表明,关键步骤IMDAV反应通过外过渡态进行,导致目标杂环的单一非对映异构体的排他性形成。获得的功能取代的噻吩并[2,3- f ]异吲哚羧酸是用于进一步转化和生物筛选的潜在有用底物。对所得化合物的抗菌评价表明,1-氧代-2-(3-(三氟甲基)苯基)六氢苯并[4,5]噻吩并[2,3- f ]异吲哚-10-甲酸是该化合物中活性最高的样品。合成文库。它对革兰氏阳性菌敏感菌株,包括金黄色葡萄球菌、屎肠球菌、蜡样芽孢杆菌和藤黄微球菌,以及革兰氏阴性菌大肠杆菌和荧光假单胞菌具有抗菌活性,MIC值范围为4至64μg·mL -1。9-Oxo-8-苯基八氢萘并[2,1- d ]噻吩并[2,3 - f ]异吲哚-10-羧酸对酵母培养物白色念珠菌具有抗真菌活性,MIC 值为 32 μM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号