当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrasound-augmented enzyodynamic-Ca2+ overload synergetic tumor nanotherapy
Biomaterials ( IF 12.8 ) Pub Date : 2024-02-26 , DOI: 10.1016/j.biomaterials.2024.122513 Meiqi Chang 1 , Lu Zhang 2 , Tingting Zhang 3 , Yanqiu Duan 1 , Wei Feng 4 , Shaoling Yang 5 , Yu Chen 4 , Zeyu Wang 4
Biomaterials ( IF 12.8 ) Pub Date : 2024-02-26 , DOI: 10.1016/j.biomaterials.2024.122513 Meiqi Chang 1 , Lu Zhang 2 , Tingting Zhang 3 , Yanqiu Duan 1 , Wei Feng 4 , Shaoling Yang 5 , Yu Chen 4 , Zeyu Wang 4
Affiliation
|
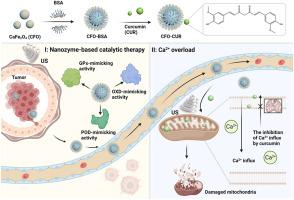
The excessive intracellular Ca2+ can induce oxidative stress, mitochondrial damage and cell apoptosis, which has been extensively explored for tumor therapy. However, the low Ca2+ accumulation originated from Ca2+ -based nanosystems substantially weakens the therapeutic effect. Herein, a functional plant polyphenol-appended enzyodynamic nanozyme system CaFe2 O4 @BSA-curcumin (abbreviation as CFO-CUR) has been rationally designed and engineered to achieve magnified Ca2+ accumulation process, deleterious reactive oxygen species (ROS) production, as well as mitochondrial dysfunction through enzyodynamic-Ca2+ overload synergistic effect. The exogenous Ca2+ released by CaFe2 O4 nanozymes under the weakly acidic tumor microenvironment and Ca2+ efflux inhibition by curcumin boost mitochondria-dominant antineoplastic efficiency. The presence of Fe components with multivalent characteristic depletes endogenous glutathione and outputs the incremental ROS due to the oxidase-, peroxidase-, glutathione peroxidase-mimicking activities. The ROS burst-triggered regulation of Ca2+ channels and pumps strengthens the intracellular Ca2+ accumulation. Especially, the exogenous ultrasound stimulation further amplifies mitochondrial damage. Both in vitro and in vivo experimental results affirm the ultrasound-augmented enzyodynamic-Ca2+ overload synergetic tumor inhibition outcomes. This study highlights the role of ultrasound coupled with functional nanozyme in the homeostasis imbalance and function disorder of mitochondria for highly efficient tumor treatment.
中文翻译:

超声增强酶动力-Ca2+超载协同肿瘤纳米疗法
细胞内过量的Ca2+可诱导氧化应激、线粒体损伤和细胞凋亡,这在肿瘤治疗中已被广泛探索。然而,源自Ca2+纳米系统的低Ca2+积累大大削弱了治疗效果。在此,合理设计和改造了功能性植物多酚酶动力纳米酶系统CaFe2O4@BSA-姜黄素(缩写为CFO-CUR),以实现放大的Ca2+积累过程、有害活性氧(ROS)的产生以及线粒体功能障碍通过酶动力-Ca2+超载协同作用。 CaFe2O4纳米酶在弱酸性肿瘤微环境下释放的外源Ca2+和姜黄素对Ca2+外流的抑制提高了线粒体主导的抗肿瘤效率。具有多价特征的 Fe 组分的存在会消耗内源性谷胱甘肽,并由于氧化酶、过氧化物酶、谷胱甘肽过氧化物酶模拟活性而输出增量 ROS。 ROS 爆发触发的 Ca2+ 通道和泵调节增强了细胞内 Ca2+ 的积累。尤其是外源性超声刺激进一步放大了线粒体损伤。体外和体内实验结果均证实了超声增强酶动力-Ca2+超载协同肿瘤抑制效果。这项研究强调了超声联合功能性纳米酶在线粒体稳态失衡和功能紊乱中的作用,从而实现高效肿瘤治疗。
更新日期:2024-02-26
中文翻译:

超声增强酶动力-Ca2+超载协同肿瘤纳米疗法
细胞内过量的Ca2+可诱导氧化应激、线粒体损伤和细胞凋亡,这在肿瘤治疗中已被广泛探索。然而,源自Ca2+纳米系统的低Ca2+积累大大削弱了治疗效果。在此,合理设计和改造了功能性植物多酚酶动力纳米酶系统CaFe2O4@BSA-姜黄素(缩写为CFO-CUR),以实现放大的Ca2+积累过程、有害活性氧(ROS)的产生以及线粒体功能障碍通过酶动力-Ca2+超载协同作用。 CaFe2O4纳米酶在弱酸性肿瘤微环境下释放的外源Ca2+和姜黄素对Ca2+外流的抑制提高了线粒体主导的抗肿瘤效率。具有多价特征的 Fe 组分的存在会消耗内源性谷胱甘肽,并由于氧化酶、过氧化物酶、谷胱甘肽过氧化物酶模拟活性而输出增量 ROS。 ROS 爆发触发的 Ca2+ 通道和泵调节增强了细胞内 Ca2+ 的积累。尤其是外源性超声刺激进一步放大了线粒体损伤。体外和体内实验结果均证实了超声增强酶动力-Ca2+超载协同肿瘤抑制效果。这项研究强调了超声联合功能性纳米酶在线粒体稳态失衡和功能紊乱中的作用,从而实现高效肿瘤治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号