当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mannosylated STING Agonist Drugamers for Dendritic Cell-Mediated Cancer Immunotherapy
ACS Central Science ( IF 18.2 ) Pub Date : 2024-02-23 , DOI: 10.1021/acscentsci.3c01310 Dinh Chuong Nguyen 1 , Kefan Song 2 , Simbarashe Jokonya 2 , Omeed Yazdani 2 , Drew L. Sellers 2 , Yonghui Wang 2 , ABM Zakaria 2 , Suzie H. Pun 1, 2 , Patrick S. Stayton 1, 2
ACS Central Science ( IF 18.2 ) Pub Date : 2024-02-23 , DOI: 10.1021/acscentsci.3c01310 Dinh Chuong Nguyen 1 , Kefan Song 2 , Simbarashe Jokonya 2 , Omeed Yazdani 2 , Drew L. Sellers 2 , Yonghui Wang 2 , ABM Zakaria 2 , Suzie H. Pun 1, 2 , Patrick S. Stayton 1, 2
Affiliation
|
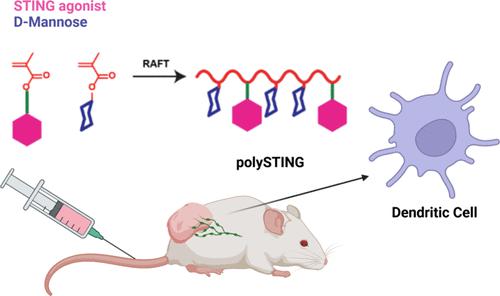
The Stimulator of Interferon Genes (STING) pathway is a promising target for cancer immunotherapy. Despite recent advances, therapies targeting the STING pathway are often limited by routes of administration, suboptimal STING activation, or off-target toxicity. Here, we report a dendritic cell (DC)-targeted polymeric prodrug platform (polySTING) that is designed to optimize intracellular delivery of a diamidobenzimidazole (diABZI) small-molecule STING agonist while minimizing off-target toxicity after parenteral administration. PolySTING incorporates mannose targeting ligands as a comonomer, which facilitates its uptake in CD206+/mannose receptor+ professional antigen-presenting cells (APCs) in the tumor microenvironment (TME). The STING agonist is conjugated through a cathepsin B-cleavable valine-alanine (VA) linker for selective intracellular drug release after receptor-mediated endocytosis. When administered intravenously in tumor-bearing mice, polySTING selectively targeted CD206+/mannose receptor+ APCs in the TME, resulting in increased cross-presenting CD8+ DCs, infiltrating CD8+ T cells in the TME as well as maturation across multiple DC subtypes in the tumor-draining lymph node (TDLN). Systemic administration of polySTING slowed tumor growth in a B16-F10 murine melanoma model as well as a 4T1 murine breast cancer model with an acceptable safety profile. Thus, we demonstrate that polySTING delivers STING agonists to professional APCs after systemic administration, generating efficacious DC-driven antitumor immunity with minimal side effects. This new polymeric prodrug platform may offer new opportunities for combining efficient targeted STING agonist delivery with other selective tumor therapeutic strategies.
中文翻译:

用于树突状细胞介导的癌症免疫治疗的甘露糖化 STING 激动剂药物药物
干扰素基因刺激器(STING)途径是癌症免疫治疗的一个有前景的靶点。尽管最近取得了进展,但针对 STING 通路的治疗通常受到给药途径、次优 STING 激活或脱靶毒性的限制。在这里,我们报告了一种树突状细胞(DC)靶向的聚合物前药平台(polySTING),该平台旨在优化二氨基苯并咪唑(diABZI)小分子STING激动剂的细胞内递送,同时最大限度地减少胃肠外给药后的脱靶毒性。 PolySTING 将甘露糖靶向配体作为共聚单体,促进其在肿瘤微环境 (TME) 中的 CD206 + /甘露糖受体+专业抗原呈递细胞 (APC) 中的摄取。 STING 激动剂通过组织蛋白酶 B 可裂解的缬氨酸-丙氨酸 (VA) 连接体缀合,用于在受体介导的内吞作用后选择性地释放细胞内药物。当对荷瘤小鼠进行静脉注射时,polySTING 选择性靶向 TME 中的 CD206 + /甘露糖受体+ APC,导致交叉呈递 CD8 + DC 增加,浸润 TME 中的 CD8 + T 细胞以及跨多种 DC 亚型的成熟。肿瘤引流淋巴结(TDLN)。在 B16-F10 小鼠黑色素瘤模型和 4T1 小鼠乳腺癌模型中,全身施用 PolySTING 可减缓肿瘤生长,且安全性可接受。因此,我们证明,polySTING 在全身给药后将 STING 激动剂递送至专业 APC,产生有效的 DC 驱动的抗肿瘤免疫,且副作用最小。这种新的聚合物前药平台可能为将有效的靶向 STING 激动剂递送与其他选择性肿瘤治疗策略相结合提供新的机会。
更新日期:2024-02-23
中文翻译:

用于树突状细胞介导的癌症免疫治疗的甘露糖化 STING 激动剂药物药物
干扰素基因刺激器(STING)途径是癌症免疫治疗的一个有前景的靶点。尽管最近取得了进展,但针对 STING 通路的治疗通常受到给药途径、次优 STING 激活或脱靶毒性的限制。在这里,我们报告了一种树突状细胞(DC)靶向的聚合物前药平台(polySTING),该平台旨在优化二氨基苯并咪唑(diABZI)小分子STING激动剂的细胞内递送,同时最大限度地减少胃肠外给药后的脱靶毒性。 PolySTING 将甘露糖靶向配体作为共聚单体,促进其在肿瘤微环境 (TME) 中的 CD206 + /甘露糖受体+专业抗原呈递细胞 (APC) 中的摄取。 STING 激动剂通过组织蛋白酶 B 可裂解的缬氨酸-丙氨酸 (VA) 连接体缀合,用于在受体介导的内吞作用后选择性地释放细胞内药物。当对荷瘤小鼠进行静脉注射时,polySTING 选择性靶向 TME 中的 CD206 + /甘露糖受体+ APC,导致交叉呈递 CD8 + DC 增加,浸润 TME 中的 CD8 + T 细胞以及跨多种 DC 亚型的成熟。肿瘤引流淋巴结(TDLN)。在 B16-F10 小鼠黑色素瘤模型和 4T1 小鼠乳腺癌模型中,全身施用 PolySTING 可减缓肿瘤生长,且安全性可接受。因此,我们证明,polySTING 在全身给药后将 STING 激动剂递送至专业 APC,产生有效的 DC 驱动的抗肿瘤免疫,且副作用最小。这种新的聚合物前药平台可能为将有效的靶向 STING 激动剂递送与其他选择性肿瘤治疗策略相结合提供新的机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号