当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing the stability of photocatalytic systems for hydrogen evolution in water by using a tris-phenyl-phenanthroline sulfonate ruthenium photosensitizer
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2024-02-23 , DOI: 10.1039/d3se01556d Fakourou Camara 1, 2 , Juan S. Aguirre-Araque 1 , Jérôme Fortage 1 , Marie-Noëlle Collomb 1
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2024-02-23 , DOI: 10.1039/d3se01556d Fakourou Camara 1, 2 , Juan S. Aguirre-Araque 1 , Jérôme Fortage 1 , Marie-Noëlle Collomb 1
Affiliation
|
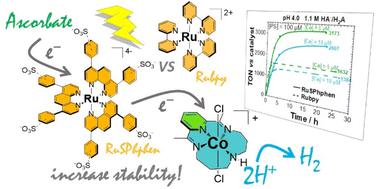
The family of ruthenium tris-bipyridine complexes remains among the most widely used molecular photosensitizers (PSs) to drive catalytic reactions using the energy of light. However, the main drawback of such PSs is the poor stability of their oxidized and reduced forms subject to ligand dissociation, especially in water that causes relatively fast deactivation of the photocatalytic systems. We were able to improve the stability and efficiency of a Ru based photocatalytic system for hydrogen production in water by using the water-soluble Na4[Ru((SO3Ph)2phen)3] derivative (RuSPhphen, (SO3Ph)2phen = disodium (1,10-phenanthroline-4,7-diyl)bis(benzenesulfonate)) in place of regular [RuII(bpy)]3Cl2 (Rubpy, bpy = 2,2′-bipyridine) PS. RuSPhphen was tested with the [CoIII(CR14)Cl2]Cl (Co) catalyst and ascorbate (HA−) as a sacrificial electron donor under visible-light irradiation. The RuSPhphen absorption coefficient being twice as high compared to Rubpy and the excited-state lifetime being much longer, while keeping almost similar potentials, more efficient intermolecular electron transfers have been observed allowing the concentration of Ru PSs to decrease by 5-fold, i.e. to 100 μM, compared to previous studies with the Rubpy/Co/HA−/H2A photocatalytic system. A substantially enhanced H2-evolving photocatalytic activity was obtained with RuSPhphen directly correlated to its better stability over Rubpy. With 1.1 M H2A/HA−, at catalyst concentrations of 10 an 5 μM, the H2 production is two times higher compared to that obtained with Rubpy. When the H2A/HA− concentration is decreased to 0.1 M, the stability of both Ru PSs is further improved although RuSPhphen still systematically outperforms Rubpy whatever the catalyst concentration, with TONs reaching up to 4770 at 5 μM catalyst. A faster electron transfer of the RuSPhphen excited state to HA− has been observed by time-resolved luminescence compared to that of Rubpy, that could be ascribed to its much longer lifetime. In addition, a much higher rate constant for back electron transfer from the RuSPhphen˙− reduced state to HA˙ was determined by nanosecond transient absorption spectroscopy that could contribute to the higher stability of RuSPhphen during photocatalysis. The greater stability of RuSPhphen over Rubpy could also be correlated to the geometry of the SPhphen ligand that makes it less prone to dissociation in water in its reduced state.
中文翻译:

使用三苯基菲咯啉磺酸钌光敏剂提高水中析氢光催化系统的稳定性
三联吡啶钌配合物家族仍然是最广泛使用的分子光敏剂(PS)之一,用于利用光能驱动催化反应。然而,此类PS的主要缺点是其氧化和还原形式的稳定性差,容易发生配体解离,特别是在水中,导致光催化系统相对快速失活。通过使用水溶性 Na 4 [Ru((SO 3 Ph) 2 phen) 3 ] 衍生物 ( RuSPhphen , (SO 3 Ph) ,我们能够提高基于 Ru 的光催化系统在水中制氢的稳定性和效率2 phen = 二钠 (1,10-菲咯啉-4,7-二基)双(苯磺酸)) 代替常规的 [Ru II (bpy)] 3 Cl 2 ( Rubpy , bpy = 2,2′-联吡啶) PS。在可见光照射下,使用 [Co III (CR14)Cl 2 ]Cl ( Co ) 催化剂和抗坏血酸 (HA - ) 作为牺牲电子供体对RuSPhphen进行了测试。RuSPhphen吸收系数是Rubpy的两倍,并且激发态寿命更长,同时保持几乎相似的电势,观察到更有效的分子间电子转移,使 Ru PSs 的浓度降低了 5倍,即100 μM,与之前使用Rubpy / Co /HA − /H 2 A 光催化系统的研究相比。RuSPhphen获得了显着增强的 H 2光催化活性,这与其比Rubpy更好的稳定性直接相关。对于 1.1 MH 2 A/HA -,在催化剂浓度为 10 和 5 μM 时,H 2产量是用Rubpy获得的产量的两倍。当 H 2 A/HA -浓度降低至 0.1 M 时,两种 Ru PS 的稳定性都进一步提高,尽管无论催化剂浓度如何, RuSPhphen仍然系统地优于Rubpy,在 5 μM 催化剂下 TON 高达 4770。与Rubpy相比,通过时间分辨发光观察到RuSPhphen激发态到 HA −的电子转移速度更快,这可以归因于其更长的使用寿命。此外,纳秒瞬态吸收光谱确定了从RuSPhphen ˙ -还原态到 HA˙ 的反电子转移速率常数要高得多,这可能有助于提高RuSPhphen在光催化过程中的稳定性。RuSPhphen比Rubpy更高的稳定性也可能与 SPhphen 配体的几何形状有关,这使得它在还原态下不易在水中解离。
更新日期:2024-02-23
中文翻译:

使用三苯基菲咯啉磺酸钌光敏剂提高水中析氢光催化系统的稳定性
三联吡啶钌配合物家族仍然是最广泛使用的分子光敏剂(PS)之一,用于利用光能驱动催化反应。然而,此类PS的主要缺点是其氧化和还原形式的稳定性差,容易发生配体解离,特别是在水中,导致光催化系统相对快速失活。通过使用水溶性 Na 4 [Ru((SO 3 Ph) 2 phen) 3 ] 衍生物 ( RuSPhphen , (SO 3 Ph) ,我们能够提高基于 Ru 的光催化系统在水中制氢的稳定性和效率2 phen = 二钠 (1,10-菲咯啉-4,7-二基)双(苯磺酸)) 代替常规的 [Ru II (bpy)] 3 Cl 2 ( Rubpy , bpy = 2,2′-联吡啶) PS。在可见光照射下,使用 [Co III (CR14)Cl 2 ]Cl ( Co ) 催化剂和抗坏血酸 (HA - ) 作为牺牲电子供体对RuSPhphen进行了测试。RuSPhphen吸收系数是Rubpy的两倍,并且激发态寿命更长,同时保持几乎相似的电势,观察到更有效的分子间电子转移,使 Ru PSs 的浓度降低了 5倍,即100 μM,与之前使用Rubpy / Co /HA − /H 2 A 光催化系统的研究相比。RuSPhphen获得了显着增强的 H 2光催化活性,这与其比Rubpy更好的稳定性直接相关。对于 1.1 MH 2 A/HA -,在催化剂浓度为 10 和 5 μM 时,H 2产量是用Rubpy获得的产量的两倍。当 H 2 A/HA -浓度降低至 0.1 M 时,两种 Ru PS 的稳定性都进一步提高,尽管无论催化剂浓度如何, RuSPhphen仍然系统地优于Rubpy,在 5 μM 催化剂下 TON 高达 4770。与Rubpy相比,通过时间分辨发光观察到RuSPhphen激发态到 HA −的电子转移速度更快,这可以归因于其更长的使用寿命。此外,纳秒瞬态吸收光谱确定了从RuSPhphen ˙ -还原态到 HA˙ 的反电子转移速率常数要高得多,这可能有助于提高RuSPhphen在光催化过程中的稳定性。RuSPhphen比Rubpy更高的稳定性也可能与 SPhphen 配体的几何形状有关,这使得它在还原态下不易在水中解离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号