当前位置:
X-MOL 学术
›
Mater. Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual-targeting nanozyme combined with aPD-L1-based immunotherapy for combating cancer recurrence and metastasis
Materials Today ( IF 24.2 ) Pub Date : 2024-02-12 , DOI: 10.1016/j.mattod.2024.01.011 Lu Tang , Yuqi Cao , Yue Yin , Hening Liu , Jingwen Feng , Cong Fu , Qingqing Zhao , Wei Wang
Materials Today ( IF 24.2 ) Pub Date : 2024-02-12 , DOI: 10.1016/j.mattod.2024.01.011 Lu Tang , Yuqi Cao , Yue Yin , Hening Liu , Jingwen Feng , Cong Fu , Qingqing Zhao , Wei Wang
|
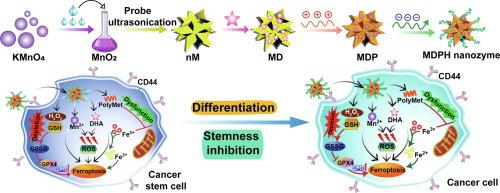
The heterogeneity of tumor microenvironment (TME) and existence of cancer stem cells (CSCs) severely impede the therapeutic efficacy of current anti-tumor treatments, which is regarded as the culprit of tumor recurrence and metastasis. Herein, a TME-responsive nanoplatform based on manganese dioxide (MnO) nanozyme was proposed for synergistic anti-tumor therapy. Dihydroartemisinin (DHA), an effective ferroptosis-inducer that possesses good reactive oxygen species-producing ability and immunological cell death-triggering property, was loaded in hollow MnO nanoparticles, which was further coated with polymetformin/hyaluronic acid (HA) to obtain MDPH nanozyme. Due to the catalase-like features of MnO nanozyme, more oxygen was produced while more glutathione was consumed in TME, facilitating DHA-mediated chemodynamic therapy (CDT). Meanwhile, MDPH nanozyme specifically targeted both tumor cells and CSCs owing to the active targetability of HA towards CD44 receptor. The ferroptosis induced by MDPH nanozyme efficaciously inhibited CSCs that are more sensitive to ferroptotic therapy but often resistant to conventional treatment regimens. By combining with aPD-L1-based immunotherapy, MDPH nanozyme efficiently suppressed 4 T1 breast tumor growth, decreased tumor stemness and reshaped immunosuppressive TME, remarkably preventing tumor relapse and metastasis. Therefore, this strategy provides a perspective and feasible approach for precise anti-tumor therapy through focusing on CSCs elimination and combined CDT/immunotherapy.
中文翻译:

双靶向纳米酶联合基于aPD-L1的免疫疗法对抗癌症复发和转移
肿瘤微环境(TME)的异质性和癌症干细胞(CSC)的存在严重阻碍了当前抗肿瘤治疗的疗效,被认为是肿瘤复发和转移的罪魁祸首。在此,提出了一种基于二氧化锰(MnO)纳米酶的 TME 响应纳米平台,用于协同抗肿瘤治疗。双氢青蒿素(DHA)是一种有效的铁死亡诱导剂,具有良好的活性氧产生能力和免疫细胞死亡触发特性,将其负载在空心MnO纳米粒子中,并进一步用聚二甲双胍/透明质酸(HA)包覆以获得MDPH纳米酶。由于MnO纳米酶具有类似过氧化氢酶的特性,TME中会产生更多的氧气,同时消耗更多的谷胱甘肽,从而促进DHA介导的化学动力学疗法(CDT)。同时,由于HA对CD44受体的主动靶向性,MDPH纳米酶特异性靶向肿瘤细胞和CSC。 MDPH 纳米酶诱导的铁死亡可有效抑制对铁死亡治疗更敏感但通常对常规治疗方案有抵抗力的 CSC。通过与基于aPD-L1的免疫疗法相结合,MDPH纳米酶有效抑制4T1乳腺肿瘤生长,降低肿瘤干性并重塑免疫抑制性TME,显着预防肿瘤复发和转移。因此,该策略为以CSC消除为重点、CDT/免疫联合治疗的精准抗肿瘤治疗提供了前景和可行的方法。
更新日期:2024-02-12
中文翻译:

双靶向纳米酶联合基于aPD-L1的免疫疗法对抗癌症复发和转移
肿瘤微环境(TME)的异质性和癌症干细胞(CSC)的存在严重阻碍了当前抗肿瘤治疗的疗效,被认为是肿瘤复发和转移的罪魁祸首。在此,提出了一种基于二氧化锰(MnO)纳米酶的 TME 响应纳米平台,用于协同抗肿瘤治疗。双氢青蒿素(DHA)是一种有效的铁死亡诱导剂,具有良好的活性氧产生能力和免疫细胞死亡触发特性,将其负载在空心MnO纳米粒子中,并进一步用聚二甲双胍/透明质酸(HA)包覆以获得MDPH纳米酶。由于MnO纳米酶具有类似过氧化氢酶的特性,TME中会产生更多的氧气,同时消耗更多的谷胱甘肽,从而促进DHA介导的化学动力学疗法(CDT)。同时,由于HA对CD44受体的主动靶向性,MDPH纳米酶特异性靶向肿瘤细胞和CSC。 MDPH 纳米酶诱导的铁死亡可有效抑制对铁死亡治疗更敏感但通常对常规治疗方案有抵抗力的 CSC。通过与基于aPD-L1的免疫疗法相结合,MDPH纳米酶有效抑制4T1乳腺肿瘤生长,降低肿瘤干性并重塑免疫抑制性TME,显着预防肿瘤复发和转移。因此,该策略为以CSC消除为重点、CDT/免疫联合治疗的精准抗肿瘤治疗提供了前景和可行的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号