当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Light-Mediated Enhancement of Glucose-Stimulated Insulin Release of Optogenetically Engineered Human Pancreatic Beta-Cells
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2024-02-20 , DOI: 10.1021/acssynbio.3c00653 Zijing Chen 1 , Demetrios M. Stoukides 1 , Emmanuel S. Tzanakakis 1, 2, 3, 4
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2024-02-20 , DOI: 10.1021/acssynbio.3c00653 Zijing Chen 1 , Demetrios M. Stoukides 1 , Emmanuel S. Tzanakakis 1, 2, 3, 4
Affiliation
|
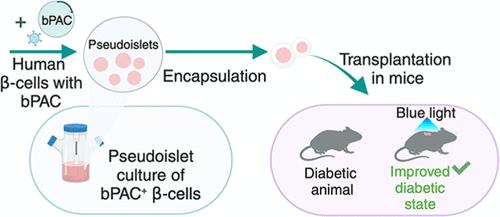
Enhancement of glucose-stimulated insulin secretion (GSIS) in exogenously delivered pancreatic β-cells is desirable, for example, to overcome the insulin resistance manifested in type 2 diabetes or to reduce the number of β-cells for supporting homeostasis of blood sugar in type 1 diabetes. Optogenetically engineered cells can potentiate their function with exposure to light. Given that cyclic adenosine monophosphate (cAMP) mediates GSIS, we surmised that optoamplification of GSIS is feasible in human β-cells carrying a photoactivatable adenylyl cyclase (PAC). To this end, human EndoC-βH3 cells were engineered to express a blue-light-activated PAC, and a workflow was established combining the scalable manufacturing of pseudoislets (PIs) with efficient adenoviral transduction, resulting in over 80% of cells carrying PAC. Changes in intracellular cAMP and GSIS were determined with the photoactivation of PAC in vitro as well as after encapsulation and implantation in mice with streptozotocin-induced diabetes. cAMP rapidly rose in β-cells expressing PAC with illumination and quickly declined upon its termination. Light-induced amplification in cAMP was concomitant with a greater than 2-fold GSIS vs β-cells without PAC in elevated glucose. The enhanced GSIS retained its biphasic pattern, and the rate of oxygen consumption remained unchanged. Diabetic mice receiving the engineered β-cell PIs exhibited improved glucose tolerance upon illumination compared to those kept in the dark or not receiving cells. The findings support the use of optogenetics for molecular customization of the β-cells toward better treatments for diabetes without the adverse effects of pharmacological approaches.
中文翻译:

光介导增强光遗传学工程人胰腺β细胞的葡萄糖刺激胰岛素释放
外源递送的胰腺 β 细胞中葡萄糖刺激的胰岛素分泌 (GSIS) 的增强是可取的,例如,为了克服 2 型糖尿病中表现出的胰岛素抵抗或减少 2 型糖尿病中支持血糖稳态的 β 细胞数量1 糖尿病。光遗传学工程细胞可以通过暴露在光下增强其功能。鉴于环磷酸腺苷 (cAMP) 介导 GSIS,我们推测 GSIS 的光放大在携带光激活腺苷酸环化酶 (PAC) 的人类 β 细胞中是可行的。为此,我们对人类 EndoC-βH3 细胞进行了改造,使其能够表达蓝光激活的 PAC,并建立了将伪胰岛 (PI) 的可扩展制造与高效腺病毒转导相结合的工作流程,从而使超过 80% 的细胞携带 PAC。细胞内 cAMP 和 GSIS 的变化是通过 PAC 的体外光激活以及在链脲佐菌素诱导的糖尿病小鼠体内封装和植入后测定的。在光照下表达 PAC 的 β 细胞中 cAMP 迅速升高,并在其终止时迅速下降。在升高的葡萄糖中,与没有 PAC 的 β 细胞相比,光诱导的 cAMP 扩增伴随着大于 2 倍的 GSIS。增强的 GSIS 保留其双相模式,耗氧率保持不变。与那些置于黑暗中或未接受细胞的小鼠相比,接受工程化 β 细胞 PI 的糖尿病小鼠在光照后表现出更好的葡萄糖耐量。研究结果支持使用光遗传学对 β 细胞进行分子定制,以更好地治疗糖尿病,而不会产生药理学方法的副作用。
更新日期:2024-02-20
中文翻译:

光介导增强光遗传学工程人胰腺β细胞的葡萄糖刺激胰岛素释放
外源递送的胰腺 β 细胞中葡萄糖刺激的胰岛素分泌 (GSIS) 的增强是可取的,例如,为了克服 2 型糖尿病中表现出的胰岛素抵抗或减少 2 型糖尿病中支持血糖稳态的 β 细胞数量1 糖尿病。光遗传学工程细胞可以通过暴露在光下增强其功能。鉴于环磷酸腺苷 (cAMP) 介导 GSIS,我们推测 GSIS 的光放大在携带光激活腺苷酸环化酶 (PAC) 的人类 β 细胞中是可行的。为此,我们对人类 EndoC-βH3 细胞进行了改造,使其能够表达蓝光激活的 PAC,并建立了将伪胰岛 (PI) 的可扩展制造与高效腺病毒转导相结合的工作流程,从而使超过 80% 的细胞携带 PAC。细胞内 cAMP 和 GSIS 的变化是通过 PAC 的体外光激活以及在链脲佐菌素诱导的糖尿病小鼠体内封装和植入后测定的。在光照下表达 PAC 的 β 细胞中 cAMP 迅速升高,并在其终止时迅速下降。在升高的葡萄糖中,与没有 PAC 的 β 细胞相比,光诱导的 cAMP 扩增伴随着大于 2 倍的 GSIS。增强的 GSIS 保留其双相模式,耗氧率保持不变。与那些置于黑暗中或未接受细胞的小鼠相比,接受工程化 β 细胞 PI 的糖尿病小鼠在光照后表现出更好的葡萄糖耐量。研究结果支持使用光遗传学对 β 细胞进行分子定制,以更好地治疗糖尿病,而不会产生药理学方法的副作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号