当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diversity Oriented Synthesis of Novel Xanthones Reveal Potent Doxorubicin-Inspired Analogs
ChemMedChem ( IF 3.4 ) Pub Date : 2024-02-13 , DOI: 10.1002/cmdc.202400055 Jonas W. Meringdal 1 , Leon Bade 2 , Gerd Bendas 2 , Dirk Menche 3
ChemMedChem ( IF 3.4 ) Pub Date : 2024-02-13 , DOI: 10.1002/cmdc.202400055 Jonas W. Meringdal 1 , Leon Bade 2 , Gerd Bendas 2 , Dirk Menche 3
Affiliation
|
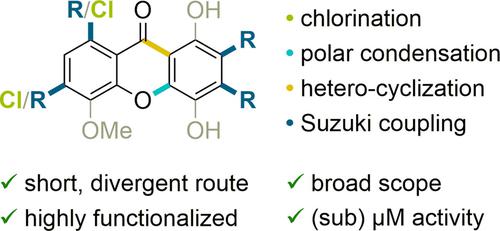
Potent but ill researched. Despite promising antiproliferative data of substituted xanthones, their structure-activity relationship remains largely unknown. We systematically synthesized previously inaccessible phenyl- and chloro-bearing xanthones through a modular chlorination followed by a concise and optimized sequence of polar condensation, cyclization and Suzuki coupling. Despite their simplicity several of these xanthones exhibited antiproliferative activities comparable to anticancer drug doxorubicin.
中文翻译:

新型氧杂蒽酮的多样性导向合成揭示了有效的阿霉素类似物
有效但研究不足。尽管取代氧杂蒽酮的抗增殖数据有希望,但它们的结构-活性关系仍然很大程度上未知。我们通过模块化氯化,然后是简洁且优化的极性缩合、环化和铃木偶联序列,系统地合成了以前无法获得的含苯基和含氯的呫吨酮。尽管这些氧杂蒽酮很简单,但其中几种表现出与抗癌药物阿霉素相当的抗增殖活性。
更新日期:2024-02-13
中文翻译:

新型氧杂蒽酮的多样性导向合成揭示了有效的阿霉素类似物
有效但研究不足。尽管取代氧杂蒽酮的抗增殖数据有希望,但它们的结构-活性关系仍然很大程度上未知。我们通过模块化氯化,然后是简洁且优化的极性缩合、环化和铃木偶联序列,系统地合成了以前无法获得的含苯基和含氯的呫吨酮。尽管这些氧杂蒽酮很简单,但其中几种表现出与抗癌药物阿霉素相当的抗增殖活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号