当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis and antiproliferative activity of allo-gibberic acid-based 1,3-aminoalcohol regioisomers
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-02-12 , DOI: 10.1039/d3md00665d Zein Alabdeen Khdar 1 , Tam Minh Le 1, 2 , Zsuzsanna Schelz 3 , István Zupkó 3 , Zsolt Szakonyi 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-02-12 , DOI: 10.1039/d3md00665d Zein Alabdeen Khdar 1 , Tam Minh Le 1, 2 , Zsuzsanna Schelz 3 , István Zupkó 3 , Zsolt Szakonyi 1
Affiliation
|
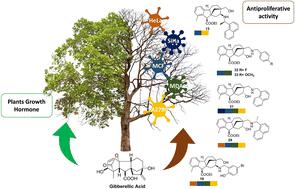
A new library of allo-gibberic acid-based aminoalcohol regioisomers was synthesised stereoselectively starting from commercially available gibberellic acid, which yields allo-gibberic acid under mild acidic conditions. The successful formation of hydroxymethyl ketone derivative 5, by acid-mediated rearrangement of previously prepared epoxide, paved the way to obtain the desired 1,3-aminoalcohols through Schiff base formation. To obtain the desired regioisomers, the primary alcohol functionality of 5 was subjected to mesylation, then replaced with either primary amine or sodium azide. The formed azide derivative was subjected to either CuAAC reaction to obtain 1,2,3-triazoles or underwent Pd-catalysed hydrogenolysis to obtain primary aminoalcohol, which was further transformed into 1,3-aminoalcohols by reductive alkylation. All prepared aminoalcohols were identified in a satisfactory manner using modern spectroscopic techniques and assessed for their antiproliferative activity against a panel of human cancer cell lines. The antiproliferative effects of the prepared compounds were assayed by in vitro MTT method against a panel of human cancer cell lines (HeLa, SiHa, A2780, MCF-7 and MDA-MB-231). A significant difference was observed in the antiproliferative activity between the regioisomers. Some compounds exerted outstanding activities against the malignant cells with limited action on fibroblasts, indicating considerable cancer selectivity.
中文翻译:

基于同种异体赤霉酸的 1,3-氨基醇结构异构体的立体选择性合成及其抗增殖活性
以市售赤霉酸为原料,立体选择性地合成了基于别位赤霉酸的氨基醇区域异构体的新文库,其在温和酸性条件下产生别位赤霉酸。通过先前制备的环氧化物的酸介导重排,成功形成羟甲基酮衍生物5,为通过席夫碱形成获得所需的 1,3-氨基醇铺平了道路。为了获得所需的区域异构体,将5的伯醇官能团进行甲磺化,然后用伯胺或叠氮化钠替代。形成的叠氮衍生物进行CuAAC反应得到1,2,3-三唑或进行Pd催化氢解得到伯氨基醇,通过还原烷基化进一步转化为1,3-氨基醇。使用现代光谱技术以令人满意的方式鉴定了所有制备的氨基醇,并评估了它们对一组人类癌细胞系的抗增殖活性。通过体外MTT 方法测定了所制备的化合物对一组人类癌细胞系(HeLa、SiHa、A2780、MCF-7 和 MDA-MB-231)的抗增殖作用。区域异构体之间的抗增殖活性存在显着差异。一些化合物对恶性细胞具有出色的活性,但对成纤维细胞的作用有限,表明具有相当大的癌症选择性。
更新日期:2024-02-12
中文翻译:

基于同种异体赤霉酸的 1,3-氨基醇结构异构体的立体选择性合成及其抗增殖活性
以市售赤霉酸为原料,立体选择性地合成了基于别位赤霉酸的氨基醇区域异构体的新文库,其在温和酸性条件下产生别位赤霉酸。通过先前制备的环氧化物的酸介导重排,成功形成羟甲基酮衍生物5,为通过席夫碱形成获得所需的 1,3-氨基醇铺平了道路。为了获得所需的区域异构体,将5的伯醇官能团进行甲磺化,然后用伯胺或叠氮化钠替代。形成的叠氮衍生物进行CuAAC反应得到1,2,3-三唑或进行Pd催化氢解得到伯氨基醇,通过还原烷基化进一步转化为1,3-氨基醇。使用现代光谱技术以令人满意的方式鉴定了所有制备的氨基醇,并评估了它们对一组人类癌细胞系的抗增殖活性。通过体外MTT 方法测定了所制备的化合物对一组人类癌细胞系(HeLa、SiHa、A2780、MCF-7 和 MDA-MB-231)的抗增殖作用。区域异构体之间的抗增殖活性存在显着差异。一些化合物对恶性细胞具有出色的活性,但对成纤维细胞的作用有限,表明具有相当大的癌症选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号