当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reductive Degradation of 4-Chloronitrobenzene by Fe(II) on Aluminum-Substituted Goethite Nanoparticles
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-02-07 , DOI: 10.1021/acsearthspacechem.3c00126 Clare M. Johnston 1 , Zachary R. Wiethorn 1 , Rebecca A. Combs 1 , Maetzin Cruz-Reyes 1 , Sawyer Lodico 1 , Hamed Nasri 2 , William A. Arnold 3 , R. Lee Penn 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-02-07 , DOI: 10.1021/acsearthspacechem.3c00126 Clare M. Johnston 1 , Zachary R. Wiethorn 1 , Rebecca A. Combs 1 , Maetzin Cruz-Reyes 1 , Sawyer Lodico 1 , Hamed Nasri 2 , William A. Arnold 3 , R. Lee Penn 1
Affiliation
|
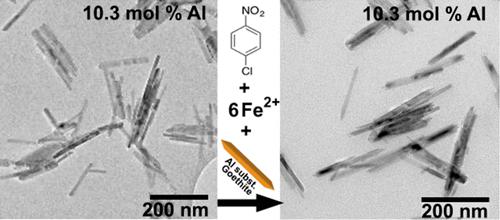
Iron oxide minerals play important roles in geo- and environmental chemistry, with substantial impacts on the chemical, physical, and (micro)biological properties of soils and also the behavior, fate, and transport of pollutants. The trivalent aluminum cation is commonly found substituted in natural iron oxides as a result of its abundance in soil and its similarity in both charge and size compared to ferric ion. Here, we examine the impact of aluminum substitution on the reactivity of Fe(II) on the iron oxide mineral goethite toward 4-chloronitrobenzene (4-ClNB), which serves as the oxidant and model pollutant. The impact of aluminum substitution on both the kinetics of contaminant reduction and the concurrent oxidative mineral growth of goethite were quantified. Rate constants were determined using both the Langmuir–Hinshelwood–Haugen–Watson (LHHW) equation and a pseudo-first-order model applied to data obtained from reactors prepared using initial concentrations of 4-ClNB from 10 to 100 μM. LHHW more accurately describes the kinetics of the reduction of 4-ClNB by Fe(II) on goethite nanoparticles and provides insights into the nature of the reactivity of goethite nanocrystals. Results demonstrate that low levels of aluminum substitution (≤4 mol % Al) result in increased reactivity, as quantified by transmission electron microscopy surface-area-normalized pseudo-first-order rate constants, and that higher levels of aluminum substitution (>4 mol % Al) result in a decrease in reactivity. In addition, aluminum substitution does not impact the surface on which the oxidative mineral growth occurs, with goethite nanoparticle lengths increasing without a concurrent increase in width observed over all particle compositions.
中文翻译:

铝取代针铁矿纳米颗粒上 Fe(II) 还原降解 4-氯硝基苯
氧化铁矿物在地质和环境化学中发挥着重要作用,对土壤的化学、物理和(微观)生物特性以及污染物的行为、归宿和迁移产生重大影响。三价铝阳离子通常在天然铁氧化物中被取代,因为它在土壤中含量丰富,并且与铁离子相比,其电荷和尺寸相似。在这里,我们研究了铝替代对氧化铁矿物针铁矿上 Fe(II) 与 4-氯硝基苯 (4-ClNB) 反应性的影响,4-氯硝基苯 (4-ClNB) 是氧化剂和模型污染物。量化了铝替代对污染物减少动力学和针铁矿同时氧化矿物生长的影响。使用 Langmuir-Hinshelwood-Haugen-Watson (LHHW) 方程和伪一阶模型确定速率常数,该模型应用于从使用 10 至 100 μM 初始浓度的 4-ClNB 制备的反应器中获得的数据。 LHHW 更准确地描述了针铁矿纳米颗粒上 Fe(II) 还原 4-ClNB 的动力学,并提供了对针铁矿纳米晶体反应性本质的见解。结果表明,低水平的铝取代(≤4 mol % Al)会导致反应性增加,如通过透射电子显微镜表面积归一化伪一级速率常数所量化,并且较高水平的铝取代(> 4 mol %Al)导致反应活性降低。此外,铝取代不会影响发生氧化矿物生长的表面,针铁矿纳米颗粒长度增加,但在所有颗粒组合物中观察到宽度没有同时增加。
更新日期:2024-02-07
中文翻译:

铝取代针铁矿纳米颗粒上 Fe(II) 还原降解 4-氯硝基苯
氧化铁矿物在地质和环境化学中发挥着重要作用,对土壤的化学、物理和(微观)生物特性以及污染物的行为、归宿和迁移产生重大影响。三价铝阳离子通常在天然铁氧化物中被取代,因为它在土壤中含量丰富,并且与铁离子相比,其电荷和尺寸相似。在这里,我们研究了铝替代对氧化铁矿物针铁矿上 Fe(II) 与 4-氯硝基苯 (4-ClNB) 反应性的影响,4-氯硝基苯 (4-ClNB) 是氧化剂和模型污染物。量化了铝替代对污染物减少动力学和针铁矿同时氧化矿物生长的影响。使用 Langmuir-Hinshelwood-Haugen-Watson (LHHW) 方程和伪一阶模型确定速率常数,该模型应用于从使用 10 至 100 μM 初始浓度的 4-ClNB 制备的反应器中获得的数据。 LHHW 更准确地描述了针铁矿纳米颗粒上 Fe(II) 还原 4-ClNB 的动力学,并提供了对针铁矿纳米晶体反应性本质的见解。结果表明,低水平的铝取代(≤4 mol % Al)会导致反应性增加,如通过透射电子显微镜表面积归一化伪一级速率常数所量化,并且较高水平的铝取代(> 4 mol %Al)导致反应活性降低。此外,铝取代不会影响发生氧化矿物生长的表面,针铁矿纳米颗粒长度增加,但在所有颗粒组合物中观察到宽度没有同时增加。



























 京公网安备 11010802027423号
京公网安备 11010802027423号