当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Biophysical Characterization of Fingolimod Derivatives as Cardiac Troponin Antagonists
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2024-02-07 , DOI: 10.1021/acsmedchemlett.3c00511 Laszlo Kondacs 1 , Priyanka Parijat 2 , Alexander J. A. Cobb 1 , Thomas Kampourakis 2
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2024-02-07 , DOI: 10.1021/acsmedchemlett.3c00511 Laszlo Kondacs 1 , Priyanka Parijat 2 , Alexander J. A. Cobb 1 , Thomas Kampourakis 2
Affiliation
|
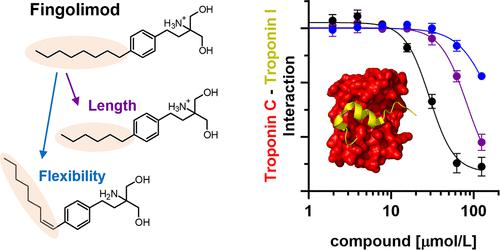
Calcium binding to cardiac troponin C (cTnC) in the thin filaments acts as a trigger for cardiac muscle contraction. The N-lobe of cTnC (NcTnC) undergoes a conformational change in the presence of calcium that allows for interaction with the switch region of cardiac troponin I (cTnISP), releasing its inhibitory effect on the thin filament structure. The small molecule fingolimod inhibits cTnC–cTnISP interactions via electrostatic repulsion between its positively charged tail and positively charged residues in cTnISP and acts as a calcium desensitizer of the contractile myofilaments. Here we investigate the structure–activity relationship of the fingolimod hydrophobic headgroup and show that increasing the alkyl chain length increases both its affinity for NcTnC and its inhibitory effect on the NcTnC–cTnISP interaction and that decreasing flexibility completely abolishes these effects. Strikingly, the longer derivatives have no effect on the calcium affinity of cTnC, suggesting that they act as better inhibitors.
中文翻译:

心肌肌钙蛋白拮抗剂芬戈莫德衍生物的合成和生物物理表征
钙与细丝中的心肌肌钙蛋白 C (cTnC) 结合,触发心肌收缩。cTnC (NcTnC) 的 N 叶在钙存在的情况下会发生构象变化,从而与心肌肌钙蛋白 I (cTnI SP ) 的开关区域相互作用,释放其对细丝结构的抑制作用。小分子芬戈莫德通过其带正电的尾部和 cTnI SP中带正电的残基之间的静电排斥来抑制 cTnC-cTnI SP相互作用,并充当收缩肌丝的钙脱敏剂。在这里,我们研究了芬戈莫德疏水头基的结构-活性关系,结果表明,增加烷基链长度会增加其对 NcTnC 的亲和力及其对 NcTnC-cTnI SP相互作用的抑制作用,而降低灵活性则完全消除了这些影响。引人注目的是,较长的衍生物对 cTnC 的钙亲和力没有影响,这表明它们可以作为更好的抑制剂。
更新日期:2024-02-07
中文翻译:

心肌肌钙蛋白拮抗剂芬戈莫德衍生物的合成和生物物理表征
钙与细丝中的心肌肌钙蛋白 C (cTnC) 结合,触发心肌收缩。cTnC (NcTnC) 的 N 叶在钙存在的情况下会发生构象变化,从而与心肌肌钙蛋白 I (cTnI SP ) 的开关区域相互作用,释放其对细丝结构的抑制作用。小分子芬戈莫德通过其带正电的尾部和 cTnI SP中带正电的残基之间的静电排斥来抑制 cTnC-cTnI SP相互作用,并充当收缩肌丝的钙脱敏剂。在这里,我们研究了芬戈莫德疏水头基的结构-活性关系,结果表明,增加烷基链长度会增加其对 NcTnC 的亲和力及其对 NcTnC-cTnI SP相互作用的抑制作用,而降低灵活性则完全消除了这些影响。引人注目的是,较长的衍生物对 cTnC 的钙亲和力没有影响,这表明它们可以作为更好的抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号