当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concurrent functional ultrasound imaging with graphene-based DC-coupled electrophysiology as a platform to study slow brain signals and cerebral blood flow under control and pathophysiological brain states
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2024-02-07 , DOI: 10.1039/d3nh00521f Julie Meng Zhang 1 , Eduard Masvidal-Codina 2, 3 , Diep Nguyen 1 , Xavi Illa 3, 4 , Julie Dégardin 1 , Ruben Goulet 1 , Elisabet Prats-Alfonso 3, 4 , Stratis Matsoukis 5, 6 , Christoph Guger 5 , Jose Antonio Garrido 2, 7 , Serge Picaud 1 , Anton Guimerà-Brunet 3, 4 , Rob C Wykes 8, 9
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2024-02-07 , DOI: 10.1039/d3nh00521f Julie Meng Zhang 1 , Eduard Masvidal-Codina 2, 3 , Diep Nguyen 1 , Xavi Illa 3, 4 , Julie Dégardin 1 , Ruben Goulet 1 , Elisabet Prats-Alfonso 3, 4 , Stratis Matsoukis 5, 6 , Christoph Guger 5 , Jose Antonio Garrido 2, 7 , Serge Picaud 1 , Anton Guimerà-Brunet 3, 4 , Rob C Wykes 8, 9
Affiliation
|
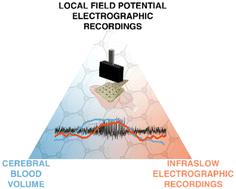
Current methodology used to investigate how shifts in brain states associated with regional cerebral blood volume (CBV) change in deep brain areas, are limited by either the spatiotemporal resolution of the CBV techniques, and/or compatibility with electrophysiological recordings; particularly in relation to spontaneous brain activity and the study of individual events. Additionally, infraslow brain signals (<0.1 Hz), including spreading depolarisations, DC-shifts and infraslow oscillations (ISO), are poorly captured by traditional AC-coupled electrographic recordings; yet these very slow brain signals can profoundly change CBV. To gain an improved understanding of how infraslow brain signals couple to CBV we present a new method for concurrent CBV with wide bandwidth electrophysiological mapping using simultaneous functional ultrasound imaging (fUS) and graphene-based field effect transistor (gFET) DC-coupled electrophysiological acquisitions. To validate the feasibility of this methodology visually-evoked neurovascular coupling (NVC) responses were examined. gFET recordings are not affected by concurrent fUS imaging, and epidural placement of gFET arrays within the imaging window did not deteriorate fUS signal quality. To examine directly the impact of infra-slow potential shifts on CBV, cortical spreading depolarisations (CSDs) were induced. A biphasic pattern of decreased, followed by increased CBV, propagating throughout the ipsilateral cortex, and a delayed decrease in deeper subcortical brain regions was observed. In a model of acute seizures, CBV oscillations were observed prior to seizure initiation. Individual seizures occurred on the rising phase of both infraslow brain signal and CBV oscillations. When seizures co-occurred with CSDs, CBV responses were larger in amplitude, with delayed CBV decreases in subcortical structures. Overall, our data demonstrate that gFETs are highly compatible with fUS and allow concurrent examination of wide bandwidth electrophysiology and CBV. This graphene-enabled technological advance has the potential to improve our understanding of how infraslow brain signals relate to CBV changes in control and pathological brain states.
中文翻译:

以基于石墨烯的直流耦合电生理学为平台的同步功能超声成像,研究受控的慢脑信号和脑血流以及病理生理脑状态
目前用于研究与深部大脑区域的局部脑血容量(CBV)变化相关的大脑状态变化如何受到CBV技术的时空分辨率和/或与电生理记录的兼容性的限制的方法;特别是与自发的大脑活动和个体事件的研究有关。此外,传统的交流耦合电图记录很难捕捉到次慢脑信号(<0.1 Hz),包括扩散去极化、直流偏移和次慢振荡(ISO)。然而,这些非常缓慢的大脑信号可以深刻地改变 CBV。为了更好地理解次慢脑信号如何与 CBV 耦合,我们提出了一种使用同步功能超声成像 (fUS) 和基于石墨烯的场效应晶体管 (gFET) 直流耦合电生理采集的宽带电生理测绘并发 CBV 的新方法。为了验证这种方法的可行性,我们检查了视觉诱发的神经血管耦合(NVC)反应。 gFET 记录不受并发 fUS 成像的影响,并且 gFET 阵列在成像窗口内的硬膜外放置不会降低 fUS 信号质量。为了直接检查亚慢电位变化对 CBV 的影响,诱导了皮质扩散去极化 (CSD)。观察到 CBV 降低、随后增加的双相模式,在整个同侧皮质中传播,并且在更深的皮质下大脑区域延迟降低。在急性癫痫发作模型中,在癫痫发作之前观察到 CBV 振荡。个别癫痫发作发生在次慢脑信号和 CBV 振荡的上升阶段。 当癫痫发作与 CSD 同时发生时,CBV 反应幅度较大,皮层下结构的 CBV 下降延迟。总体而言,我们的数据表明 gFET 与 fUS 高度兼容,并允许同时检查宽带宽电生理学和 CBV。这种石墨烯驱动的技术进步有可能提高我们对次慢脑信号如何与控制和病理性大脑状态的 CBV 变化相关的理解。
更新日期:2024-02-08
中文翻译:

以基于石墨烯的直流耦合电生理学为平台的同步功能超声成像,研究受控的慢脑信号和脑血流以及病理生理脑状态
目前用于研究与深部大脑区域的局部脑血容量(CBV)变化相关的大脑状态变化如何受到CBV技术的时空分辨率和/或与电生理记录的兼容性的限制的方法;特别是与自发的大脑活动和个体事件的研究有关。此外,传统的交流耦合电图记录很难捕捉到次慢脑信号(<0.1 Hz),包括扩散去极化、直流偏移和次慢振荡(ISO)。然而,这些非常缓慢的大脑信号可以深刻地改变 CBV。为了更好地理解次慢脑信号如何与 CBV 耦合,我们提出了一种使用同步功能超声成像 (fUS) 和基于石墨烯的场效应晶体管 (gFET) 直流耦合电生理采集的宽带电生理测绘并发 CBV 的新方法。为了验证这种方法的可行性,我们检查了视觉诱发的神经血管耦合(NVC)反应。 gFET 记录不受并发 fUS 成像的影响,并且 gFET 阵列在成像窗口内的硬膜外放置不会降低 fUS 信号质量。为了直接检查亚慢电位变化对 CBV 的影响,诱导了皮质扩散去极化 (CSD)。观察到 CBV 降低、随后增加的双相模式,在整个同侧皮质中传播,并且在更深的皮质下大脑区域延迟降低。在急性癫痫发作模型中,在癫痫发作之前观察到 CBV 振荡。个别癫痫发作发生在次慢脑信号和 CBV 振荡的上升阶段。 当癫痫发作与 CSD 同时发生时,CBV 反应幅度较大,皮层下结构的 CBV 下降延迟。总体而言,我们的数据表明 gFET 与 fUS 高度兼容,并允许同时检查宽带宽电生理学和 CBV。这种石墨烯驱动的技术进步有可能提高我们对次慢脑信号如何与控制和病理性大脑状态的 CBV 变化相关的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号