当前位置:
X-MOL 学术
›
J. Supercrit. Fluids
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor-liquid equilibrium measurement and critical line prediction for carbon dioxide (CO2) + fluoroethane (R161) binary mixtures
The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2024-02-03 , DOI: 10.1016/j.supflu.2024.106205 Zirui Wu , Rui Sun , Lingfeng Shi , Peng Hu , Hua Tian , Xuan Wang , Gequn Shu
The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2024-02-03 , DOI: 10.1016/j.supflu.2024.106205 Zirui Wu , Rui Sun , Lingfeng Shi , Peng Hu , Hua Tian , Xuan Wang , Gequn Shu
|
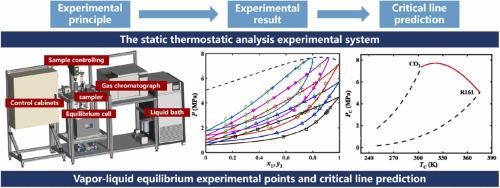
CO-based binary mixtures are excellent alternative working fluids due to their environmentally friendly and thermodynamically favorable properties. The vapor-liquid equilibrium properties of mixtures are necessary to determine the enthalpy and entropy of the dew point and bubble point. Isothermal vapor-liquid equilibrium data for the CO/R161 are measured at temperatures ranging from 283.15 K to 323.15 K and at pressures ranging from 1.28 MPa to 7.30 MPa. The temperature and pressure of the mixtures are measured using a static thermostatic analysis vapor-liquid equilibrium system. Two electromagnetic capillary samplers repeatedly collect the vapor-liquid phase components from the equilibrium cell for analysis via gas chromatograph. The standard uncertainties for mole fractions, pressure, and temperature are 0.004, 0.002 MPa, and 0.06 K, respectively. The Peng-Robinson equation, incorporating the Wong-Sandler mixing rule and the Non-Random Two-Liquid model, is utilized to fit the obtained vapor-liquid equilibrium data. The average absolute relative deviation of pressure is 0.56%, and the average absolute deviation of vapor phase molar fractions is 0.0049. The relative volatility under the five isotherms is also calculated, and most of the experimental data fall within a deviation of ± 5% from experimental values/ calculated results. In addition, this model accurately predicts the critical point line of the CO and R161 binary mixtures through the binary interaction parameters.
中文翻译:

二氧化碳(CO2)+氟乙烷(R161)二元混合物的汽液平衡测量和临界线预测
CO 基二元混合物由于其环保和热力学有利的特性,是优秀的替代工作流体。混合物的汽液平衡特性对于确定露点和泡点的焓和熵是必要的。CO/R161 的等温汽液平衡数据是在 283.15 K 至 323.15 K 的温度范围和 1.28 MPa 至 7.30 MPa 的压力范围内测量的。使用静态恒温分析汽液平衡系统测量混合物的温度和压力。两个电磁毛细管进样器重复收集平衡池中的汽液相成分,通过气相色谱仪进行分析。摩尔分数、压力和温度的标准不确定度分别为 0.004、0.002 MPa 和 0.06 K。利用结合了 Wong-Sandler 混合规则和非随机二液模型的 Peng-Robinson 方程来拟合所获得的汽液平衡数据。压力的平均绝对相对偏差为0.56%,气相摩尔分数的平均绝对偏差为0.0049。还计算了五个等温线下的相对挥发度,大部分实验数据与实验值/计算结果的偏差在±5%以内。此外,该模型通过二元相互作用参数准确预测了CO和R161二元混合物的临界点线。
更新日期:2024-02-03
中文翻译:

二氧化碳(CO2)+氟乙烷(R161)二元混合物的汽液平衡测量和临界线预测
CO 基二元混合物由于其环保和热力学有利的特性,是优秀的替代工作流体。混合物的汽液平衡特性对于确定露点和泡点的焓和熵是必要的。CO/R161 的等温汽液平衡数据是在 283.15 K 至 323.15 K 的温度范围和 1.28 MPa 至 7.30 MPa 的压力范围内测量的。使用静态恒温分析汽液平衡系统测量混合物的温度和压力。两个电磁毛细管进样器重复收集平衡池中的汽液相成分,通过气相色谱仪进行分析。摩尔分数、压力和温度的标准不确定度分别为 0.004、0.002 MPa 和 0.06 K。利用结合了 Wong-Sandler 混合规则和非随机二液模型的 Peng-Robinson 方程来拟合所获得的汽液平衡数据。压力的平均绝对相对偏差为0.56%,气相摩尔分数的平均绝对偏差为0.0049。还计算了五个等温线下的相对挥发度,大部分实验数据与实验值/计算结果的偏差在±5%以内。此外,该模型通过二元相互作用参数准确预测了CO和R161二元混合物的临界点线。



























 京公网安备 11010802027423号
京公网安备 11010802027423号