当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Removal of heavy metals by BEA zeolite/Fe3O4 composite prepared via dry-gel conversion method using agrowaste-derived raw material
Solid State Sciences ( IF 3.5 ) Pub Date : 2024-02-02 , DOI: 10.1016/j.solidstatesciences.2024.107473 Vanpaseuth Phouthavong , Takeshi Hagio , Jae-Hyeok Park , Supinya Nijpanich , Kanchanok Duangkhai , Ratana Rujiravanit , Piyatida Thaveemas , Vanseng Chounlamany , Long Kong , Liang Li , Ryoichi Ichino
Solid State Sciences ( IF 3.5 ) Pub Date : 2024-02-02 , DOI: 10.1016/j.solidstatesciences.2024.107473 Vanpaseuth Phouthavong , Takeshi Hagio , Jae-Hyeok Park , Supinya Nijpanich , Kanchanok Duangkhai , Ratana Rujiravanit , Piyatida Thaveemas , Vanseng Chounlamany , Long Kong , Liang Li , Ryoichi Ichino
|
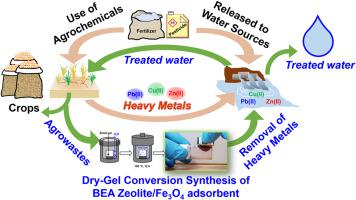
Using agrochemicals are considered as an anthropogenic source of heavy metal contamination in water, and ecofriendly remediation tools for removal is desired. Here, we demonstrate material circulation by converting SiO extracted from agrowaste: rice husk ash, into an easily collectable adsorbent: BEA zeolite/FeO composite, through dry-gel conversion technique. In addition, the synthesized BEA zeolite/FeO composite was tested for removal of heavy metals for the first time. Instrumental analyses confirmed that incorporated FeO particles inside BEA zeolites showed no severe effects on its morphologies. Pseudo-second order kinetic and Langmuir isotherm models were found best described the adsorption of three typical toxic heavy metals: Pb(II), Cu(II), Zn(II). Moderate adsorption capacities of 0.675, 0.310, and 0.308 mmol/g for Pb(II), Cu(II), and Zn(II), respectively, following the selectivity order: Pb(II) > Cu(II) ≈ Zn(II) were obtained. The composite adsorbent exhibits magnetic collectability, reusability and stability, indicating its practicality for removal of heavy metals from water.
中文翻译:

利用农业废弃物原料通过干凝胶转化法制备 BEA 沸石/Fe3O4 复合材料去除重金属
使用农用化学品被认为是水中重金属污染的人为来源,因此需要生态友好的修复工具来去除。在这里,我们通过干凝胶转化技术将从农业废弃物(稻壳灰)中提取的 SiO 转化为易于收集的吸附剂:BEA 沸石/Fe3O 复合材料,从而演示材料循环。此外,首次测试了合成的 BEA 沸石/Fe3O 复合材料的重金属去除效果。仪器分析证实,BEA 沸石中掺入的 FeO 颗粒对其形态没有严重影响。准二级动力学模型和 Langmuir 等温线模型最能描述三种典型有毒重金属的吸附:Pb(II)、Cu(II)、Zn(II)。对 Pb(II)、Cu(II) 和 Zn(II) 的中等吸附容量分别为 0.675、0.310 和 0.308 mmol/g,遵循选择性顺序:Pb(II) > Cu(II) ≈ Zn(II) ) 获得。该复合吸附剂具有磁性收集性、可重复使用性和稳定性,表明其在去除水中重金属方面具有实用性。
更新日期:2024-02-02
中文翻译:

利用农业废弃物原料通过干凝胶转化法制备 BEA 沸石/Fe3O4 复合材料去除重金属
使用农用化学品被认为是水中重金属污染的人为来源,因此需要生态友好的修复工具来去除。在这里,我们通过干凝胶转化技术将从农业废弃物(稻壳灰)中提取的 SiO 转化为易于收集的吸附剂:BEA 沸石/Fe3O 复合材料,从而演示材料循环。此外,首次测试了合成的 BEA 沸石/Fe3O 复合材料的重金属去除效果。仪器分析证实,BEA 沸石中掺入的 FeO 颗粒对其形态没有严重影响。准二级动力学模型和 Langmuir 等温线模型最能描述三种典型有毒重金属的吸附:Pb(II)、Cu(II)、Zn(II)。对 Pb(II)、Cu(II) 和 Zn(II) 的中等吸附容量分别为 0.675、0.310 和 0.308 mmol/g,遵循选择性顺序:Pb(II) > Cu(II) ≈ Zn(II) ) 获得。该复合吸附剂具有磁性收集性、可重复使用性和稳定性,表明其在去除水中重金属方面具有实用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号