当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heterogeneous interactions and transformations of dibasic esters with indoor relevant surfaces
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2024-01-26 , DOI: 10.1039/d3em00542a Cholaphan Deeleepojananan 1 , Jinxu Zhou 2 , Vicki H. Grassian 1
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2024-01-26 , DOI: 10.1039/d3em00542a Cholaphan Deeleepojananan 1 , Jinxu Zhou 2 , Vicki H. Grassian 1
Affiliation
|
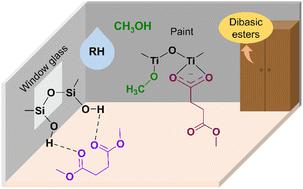
Dibasic esters (DBEs) have recently become emerging indoor air pollutants due to their usage as a solvent for mixtures of paints and coatings. In this study, we explored the adsorption/desorption kinetics, heterogeneous interactions, and chemical transformations of dimethyl succinate (DMS, C6H10O4), a component of commercial dibasic ester solvent mixtures, on indoor relevant surfaces using transmission Fourier-transform infrared (FTIR) spectroscopy and high-resolution mass spectrometry (HRMS). Silica (SiO2) and rutile (TiO2) were used as proxies for window glass, and an active component in paint and self-cleaning surfaces, respectively. FTIR spectroscopy of these surfaces shows that DMS can interact with SiO2 and TiO2 through hydrogen bonding between the carbonyl oxygen and surface hydroxyl groups. The kinetics show fast adsorption of DMS onto these surfaces followed by slow desorption. Furthermore, new products formed observed on TiO2 surfaces in addition to molecularly adsorbed DMS. In particular, succinate (C5H7O) was observed binding to the surface in a bidentate chelating coordination mode as indicated by the appearance of νas(COO−) and νs(COO−) bands in the FTIR spectra. These absorption bands grow in intensity over time and the resulting product remains strongly adsorbed on the surface. The formation of adsorbed succinate is a result of a reaction with DMS on Lewis acid sites of the TiO2 surface. Overall, the slow desorption of these adsorbed species indicates that indoor surfaces can become long term reservoirs for dibasic esters and their surface products. Moreover, in the presence of ∼50% relative humidity, water displaces outer layers of adsorbed DMS on SiO2 and TiO2, while having no impact on the more strongly bound surface species.
中文翻译:

二元酯与室内相关表面的异质相互作用和转化
二元酯 (DBE) 最近已成为新兴的室内空气污染物,因为它们用作油漆和涂料混合物的溶剂。在这项研究中,我们利用透射傅里叶变换探索了商业二元酯溶剂混合物的一种成分琥珀酸二甲酯(DMS,C 6 H 10 O 4 )在室内相关表面上的吸附/解吸动力学、非均相相互作用和化学转化。红外(FTIR)光谱和高分辨率质谱(HRMS)。二氧化硅 (SiO 2 ) 和金红石 (TiO 2 ) 分别用作窗玻璃的替代品以及油漆和自清洁表面的活性成分。这些表面的 FTIR 光谱表明,DMS 可以通过羰基氧和表面羟基之间的氢键与 SiO 2和 TiO 2相互作用。动力学表明 DMS 快速吸附到这些表面上,然后缓慢解吸。此外,除了分子吸附的DMS之外,在TiO 2表面上还观察到形成了新产物。特别是,观察到琥珀酸 (C 5 H 7 O) 以双齿螯合配位模式结合到表面,如FTIR 光谱中ν as (COO - ) 和ν s (COO - ) 谱带的出现所示。这些吸收带的强度随着时间的推移而增强,所得产品仍然强烈吸附在表面上。吸附的琥珀酸盐的形成是与DMS在TiO 2表面的路易斯酸位点上反应的结果。总体而言,这些吸附物质的缓慢解吸表明室内表面可以成为二元酯及其表面产物的长期储存库。此外,在~50%的相对湿度下,水取代了SiO 2和TiO 2上吸附的DMS的外层,同时对更强结合的表面物质没有影响。
更新日期:2024-01-26
中文翻译:

二元酯与室内相关表面的异质相互作用和转化
二元酯 (DBE) 最近已成为新兴的室内空气污染物,因为它们用作油漆和涂料混合物的溶剂。在这项研究中,我们利用透射傅里叶变换探索了商业二元酯溶剂混合物的一种成分琥珀酸二甲酯(DMS,C 6 H 10 O 4 )在室内相关表面上的吸附/解吸动力学、非均相相互作用和化学转化。红外(FTIR)光谱和高分辨率质谱(HRMS)。二氧化硅 (SiO 2 ) 和金红石 (TiO 2 ) 分别用作窗玻璃的替代品以及油漆和自清洁表面的活性成分。这些表面的 FTIR 光谱表明,DMS 可以通过羰基氧和表面羟基之间的氢键与 SiO 2和 TiO 2相互作用。动力学表明 DMS 快速吸附到这些表面上,然后缓慢解吸。此外,除了分子吸附的DMS之外,在TiO 2表面上还观察到形成了新产物。特别是,观察到琥珀酸 (C 5 H 7 O) 以双齿螯合配位模式结合到表面,如FTIR 光谱中ν as (COO - ) 和ν s (COO - ) 谱带的出现所示。这些吸收带的强度随着时间的推移而增强,所得产品仍然强烈吸附在表面上。吸附的琥珀酸盐的形成是与DMS在TiO 2表面的路易斯酸位点上反应的结果。总体而言,这些吸附物质的缓慢解吸表明室内表面可以成为二元酯及其表面产物的长期储存库。此外,在~50%的相对湿度下,水取代了SiO 2和TiO 2上吸附的DMS的外层,同时对更强结合的表面物质没有影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号