European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.ejpb.2024.114184 Yunfeng Hu , Jiahui Zou , Qianqian Wang , Yang Chen , Hui Wang , Jin Li
|
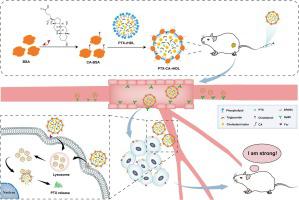
Lipoprotein-derived nanotherapeutics based on endogenous lipid supramolecules have been regarded as an exceptional and promising approach for anti-tumor drug delivery. However, certain challenges associated with the main component apolipoprotein, such as limited availability, high cost, and insufficient specificity of relevant receptor expression, pose significant barriers to its widespread development and application. The objective of this study is to fabricate lipoprotein-mimicking nanocomposites, denoted as CA-P-rHDL by substituting apolipoprotein with chenodeoxycholic acid (CA) modified bovine serum albumin (BSA), and subsequently assess their tumor-targeting capability and anti-tumor efficacy. CA modified BSA (CA-BSA) was successfully synthesized and characterized by quantifying the degree of protein substitution. Subsequently, a nanostructured lipid carrier (NLC) mimicking the hydrophobic core of natural lipoproteins was attached with CA-BSA to form a lipoprotein-mimic nanocomplex termed as CA-rHDL. CA-rHDL was endowed with lipoprotein-like structures, favorable particle size, zeta potential and excellent paclitaxel encapsulation (termed as CA-P-rHDL). The internalization of CA-rHDL by HepG2 cells exhibited significantly superior efficiency, with a notably higher in HepG2 cells compared to LO2 cells. Confocal laser scanning microscopy revealed that CA-rHDL evaded lysosomal degradation and was evenly distributed throughout the cells. CCK-8 studies demonstrated that CA-P-rHDL exhibited significantly superior inhibition of tumor cells growth compared to other paclitaxel formulations in vitro. Moreover, in vivo imaging observation in H22 tumor-bearing mouse models exhibited a rapid and consistent accumulation of CA-rHDL within tumors, while CA-P-rHDL demonstrated remarkable efficacy against cancer in these mice. These exceptional capabilities of CA-P-rHDL can be attributed to the synergistic targeting effect facilitated by the combination of CA and BSA, rendering it a promising and versatile drug delivery system for targeted anticancer therapy. Consequently, CA-P-rHDL established a highly potential platform for simulating the reconstitution of supramolecular nanovehicles.
中文翻译:

用鹅去氧胆酸修饰蛋白重构模拟脂蛋白的纳米治疗药物,以实现有效的肿瘤靶向
基于内源性脂质超分子的脂蛋白衍生纳米疗法被认为是一种特殊且有前途的抗肿瘤药物递送方法。然而,与主要成分载脂蛋白相关的某些挑战,例如可用性有限、成本高以及相关受体表达特异性不足等,对其广泛开发和应用构成了重大障碍。本研究的目的是通过用鹅去氧胆酸(CA)修饰的牛血清白蛋白(BSA)替代载脂蛋白来制备模拟脂蛋白的纳米复合材料,表示为CA-P-rHDL,并随后评估其肿瘤靶向能力和抗肿瘤功效。成功合成了CA修饰的 BSA (CA-BSA),并通过量化蛋白质取代程度进行了表征。随后,模拟天然脂蛋白疏水核心的纳米结构脂质载体(NLC)与CA-BSA连接,形成脂蛋白模拟纳米复合物,称为CA-rHDL。CA-rHDL 具有脂蛋白样结构、良好的粒径、zeta 电位和优异的紫杉醇封装性(称为 CA-P-rHDL)。HepG2 细胞对 CA-rHDL 的内化表现出显着优越的效率,与 LO2 细胞相比,HepG2 细胞的效率明显更高。共焦激光扫描显微镜显示,CA-rHDL 避免了溶酶体降解,并均匀分布在整个细胞中。CCK-8 研究表明,与其他紫杉醇制剂相比,CA-P-rHDL在体外。此外,体内成像观察显示,CA-rHDL在肿瘤内快速且一致地积累,而CA-P-rHDL在这些小鼠中表现出显着的抗癌功效。CA-P-rHDL 的这些卓越能力可归因于 CA 和 BSA 组合所促进的协同靶向效应,使其成为一种有前途且多功能的靶向抗癌治疗药物递送系统。因此,CA-P-rHDL 建立了一个非常有潜力的平台来模拟超分子纳米载体的重构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号