当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Study on the Reaction of Cuprite (Cu2O) with Acetate-Bearing Hydrothermal Fluids at 100–250 °C and 5–30 MPa
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-01-19 , DOI: 10.1021/acsearthspacechem.3c00254 Dongmei Qi 1, 2 , Harald Behrens 1 , Marina Lazarov 1 , Roman Botcharnikov 3 , Chao Zhang 4 , Christian Ostertag-Henning 5 , Stefan Weyer 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-01-19 , DOI: 10.1021/acsearthspacechem.3c00254 Dongmei Qi 1, 2 , Harald Behrens 1 , Marina Lazarov 1 , Roman Botcharnikov 3 , Chao Zhang 4 , Christian Ostertag-Henning 5 , Stefan Weyer 1
Affiliation
|
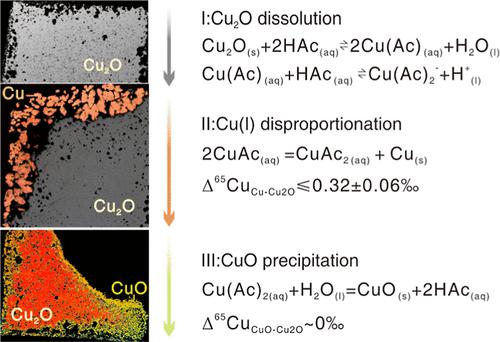
To improve our understanding of the formation of sedimentary copper deposits, the reaction of cuprite with 0.2 m HAc-KAc or pure H2O solutions is studied systematically at 100–250 °C and 5–30 MPa. The experiments were carried out for periods of up to 72 h in a Parr autoclave, allowing for the in situ sampling of the fluid phase. The experiments conducted in this study demonstrate that cuprite (Cu2O) underwent a series of changes: (i) simple dissolution, (ii) Cu(I) disproportionation to native Cu and Cu(II), and (iii) subsequent oxidation into tenorite (CuO). In pure water, only (i) and (ii) steps can be discerned, whereas all three processes have been observed in an acetate-bearing system. In HAc-KAc solutions, the maximum dissolved Cu content correlates inversely with temperature, i.e., 378 to 168 μg/g at 100 and 200 °C, respectively. However, equilibrium has not been reached in our experiments and these values may be treated as minimum cuprite solubility. In situ Cu isotope analyses have been carried out by laser ablation combined with a multicollector inductively coupled plasma-mass spectrometer. The data imply that copper isotope fractionation during cuprite replacement reactions is small. Both the microscopic observations on cross sections and the analytical data support the idea that the mineral replacement reaction is controlled by a coupled dissolution-reprecipitation (CDR) mechanism. This applies to both the deposition of metallic copper and the formation of tenorite. As suggested by the formation of pore spaces in the deposited layers, only a portion of the dissolved copper is redeposited directly in situ. The isotopic analyses of the solution and solid phases show that the partial transfer of copper into the surrounding solution is not associated with a significant isotopic effect, e.g., a measured difference between Cu and Cu2O is within 0.32 ± 0.06‰. Our study indicates that acetate plays a dual role in copper transport and deposition. On one hand, the presence of acetate strongly enhances the Cu content in solution up to 400 μg/g, implying that acetate complexation can be responsible for metal transport in hydrothermal fluids. On the other hand, decarboxylation of acetate substantially decreases the dissolved Cu and aids the precipitation of tenorite. This may lead to the co-occurrence of Cu-bearing minerals with different oxidation valence states at low temperatures in a variety of geological settings such as supergene hydrothermal systems.
中文翻译:

100~250 ℃、5~30 MPa 下赤铜矿(Cu2O)与含醋酸盐热液反应的实验研究
为了加深对沉积铜矿形成的理解,系统研究了赤铜矿与0.2 m HAc-KAc 或纯H 2 O 溶液在100–250 °C 和5–30 MPa 下的反应。实验在帕尔高压釜中进行长达 72 小时,以便对液相进行原位取样。本研究中进行的实验表明,赤铜矿 (Cu 2 O) 经历了一系列变化:(i) 简单溶解,(ii) Cu(I) 歧化为天然 Cu 和 Cu(II),以及 (iii) 随后氧化成长长岩(CuO)。在纯水中,只能辨别出 (i) 和 (ii) 步骤,而所有三个过程都在含醋酸盐的系统中观察到。在HAc-KAc 溶液中,最大溶解Cu 含量与温度成反比,即100 和200 °C 时分别为378 至168 μg/g。然而,在我们的实验中尚未达到平衡,这些值可以被视为最小赤铜矿溶解度。原位铜同位素分析是通过激光烧蚀结合多接收器电感耦合等离子体质谱仪进行的。数据表明赤铜矿置换反应期间铜同位素分馏很小。横截面的显微观察和分析数据都支持矿物置换反应是由耦合溶解-再沉淀(CDR)机制控制的观点。这适用于金属铜的沉积和镁钠长岩的形成。正如沉积层中孔隙空间的形成所表明的,只有一部分溶解的铜直接在原位重新沉积。溶液和固相的同位素分析表明,铜部分转移到周围溶液中与显着的同位素效应无关,例如,测量的Cu和Cu 2 O之间的差异在0.32±0.06‰之内。我们的研究表明乙酸盐在铜的传输和沉积中发挥双重作用。一方面,乙酸盐的存在使溶液中的 Cu 含量大幅提高至 400 μg/g,这意味着乙酸盐络合可以负责热液中的金属传输。另一方面,乙酸盐的脱羧作用显着减少了溶解的铜并有助于斑长岩的沉淀。这可能导致在各种地质环境(例如表生热液系统)中,低温下具有不同氧化价态的含铜矿物共存。
更新日期:2024-01-19
中文翻译:

100~250 ℃、5~30 MPa 下赤铜矿(Cu2O)与含醋酸盐热液反应的实验研究
为了加深对沉积铜矿形成的理解,系统研究了赤铜矿与0.2 m HAc-KAc 或纯H 2 O 溶液在100–250 °C 和5–30 MPa 下的反应。实验在帕尔高压釜中进行长达 72 小时,以便对液相进行原位取样。本研究中进行的实验表明,赤铜矿 (Cu 2 O) 经历了一系列变化:(i) 简单溶解,(ii) Cu(I) 歧化为天然 Cu 和 Cu(II),以及 (iii) 随后氧化成长长岩(CuO)。在纯水中,只能辨别出 (i) 和 (ii) 步骤,而所有三个过程都在含醋酸盐的系统中观察到。在HAc-KAc 溶液中,最大溶解Cu 含量与温度成反比,即100 和200 °C 时分别为378 至168 μg/g。然而,在我们的实验中尚未达到平衡,这些值可以被视为最小赤铜矿溶解度。原位铜同位素分析是通过激光烧蚀结合多接收器电感耦合等离子体质谱仪进行的。数据表明赤铜矿置换反应期间铜同位素分馏很小。横截面的显微观察和分析数据都支持矿物置换反应是由耦合溶解-再沉淀(CDR)机制控制的观点。这适用于金属铜的沉积和镁钠长岩的形成。正如沉积层中孔隙空间的形成所表明的,只有一部分溶解的铜直接在原位重新沉积。溶液和固相的同位素分析表明,铜部分转移到周围溶液中与显着的同位素效应无关,例如,测量的Cu和Cu 2 O之间的差异在0.32±0.06‰之内。我们的研究表明乙酸盐在铜的传输和沉积中发挥双重作用。一方面,乙酸盐的存在使溶液中的 Cu 含量大幅提高至 400 μg/g,这意味着乙酸盐络合可以负责热液中的金属传输。另一方面,乙酸盐的脱羧作用显着减少了溶解的铜并有助于斑长岩的沉淀。这可能导致在各种地质环境(例如表生热液系统)中,低温下具有不同氧化价态的含铜矿物共存。



























 京公网安备 11010802027423号
京公网安备 11010802027423号