Journal of Materiomics ( IF 9.4 ) Pub Date : 2024-01-14 , DOI: 10.1016/j.jmat.2023.12.007 Steven M. Smith , William G. Fahrenholtz , Gregory E. Hilmas , Stefano Curtarolo
|
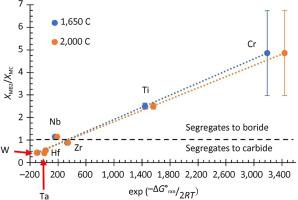
Equilibrium Gibbs' free energy calculations were used to determine metal segregation trends between boride and carbide solid solutions containing two metals that are relevant to dual phase high entropy ceramics. The model predicted that Ti had the strongest tendency to segregate to the boride phase followed by Zr, Nb, Mo, V, Hf, and Ta, which matches experimental results of measured compositions. The ratio of a metal in the carbide phase to the content of the same metal in the corresponding metal boride had a linear trend with the change in standard Gibbs' free energy of reaction for a metal carbide reacting with B4C to produce its corresponding metal boride and carbon. The proposed model was used to predict the changes in standard Gibbs' free energy for CrC→CrB2 to be −260 kJ and WC→WB2 to be 148 kJ, which indicates that Cr has the strongest segregation to the boride and W has the strongest segregation to the carbide. The proposed model can be used to estimate the segregation of metals in dual phase high entropy boride-carbide ceramics of any boride/carbide ratio or metal content.
中文翻译:

双相高熵陶瓷中金属偏析的热力学分析
平衡吉布斯自由能计算用于确定含有与双相高熵陶瓷相关的两种金属的硼化物和碳化物固溶体之间的金属偏析趋势。该模型预测 Ti 偏析为硼化物相的倾向最强,其次是 Zr、Nb、Mo、V、Hf 和 Ta,这与测量成分的实验结果相匹配。碳化物相中的金属与相应金属硼化物中同种金属的含量之比,与金属碳化物与B 4 C反应生成相应金属的标准吉布斯反应自由能的变化呈线性趋势硼化物和碳。该模型用于预测CrC→CrB 2的标准吉布斯自由能变化为-260 kJ,WC→WB 2为148 kJ,这表明Cr对硼化物的偏析最强,W对硼化物的偏析最强。对碳化物的偏析最强。所提出的模型可用于估计任何硼化物/碳化物比率或金属含量的双相高熵硼化物-碳化物陶瓷中金属的偏析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号