Cell Host & Microbe ( IF 30.3 ) Pub Date : 2024-01-11 , DOI: 10.1016/j.chom.2023.12.013 Melanie Schirmer , Martin Stražar , Julian Avila-Pacheco , Daniel F. Rojas-Tapias , Eric M. Brown , Emily Temple , Amy Deik , Kevin Bullock , Sarah Jeanfavre , Kerry Pierce , Shen Jin , Rachele Invernizzi , Marie-Madlen Pust , Zach Costliow , David R. Mack , Anne M. Griffiths , Thomas Walters , Brendan M. Boyle , Subra Kugathasan , Hera Vlamakis , Jeffrey Hyams , Lee Denson , Clary B. Clish , Ramnik J. Xavier
|
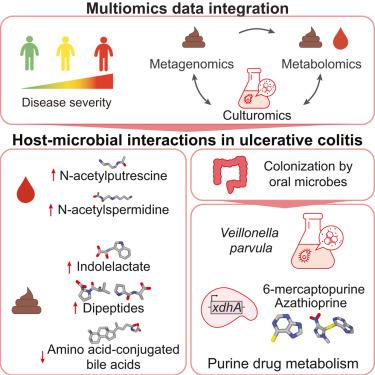
Understanding the role of the microbiome in inflammatory diseases requires the identification of microbial effector molecules. We established an approach to link disease-associated microbes to microbial metabolites by integrating paired metagenomics, stool and plasma metabolomics, and culturomics. We identified host-microbial interactions correlated with disease activity, inflammation, and the clinical course of ulcerative colitis (UC) in the Predicting Response to Standardized Colitis Therapy (PROTECT) pediatric inception cohort. In severe disease, metabolite changes included increased dipeptides and tauro-conjugated bile acids (BAs) and decreased amino-acid-conjugated BAs in stool, whereas in plasma polyamines (N-acetylputrescine and N1-acetylspermidine) increased. Using patient samples and Veillonella parvula as a model, we uncovered nitrate- and lactate-dependent metabolic pathways, experimentally linking V. parvula expansion to immunomodulatory tryptophan metabolite production. Additionally, V. parvula metabolizes immunosuppressive thiopurine drugs through xdhA xanthine dehydrogenase, potentially impairing the therapeutic response. Our findings demonstrate that the microbiome contributes to disease-associated metabolite changes, underscoring the importance of these interactions in disease pathology and treatment.
中文翻译:

将微生物基因与血浆和粪便代谢物联系起来揭示溃疡性结肠炎病程背后的宿主微生物相互作用
了解微生物组在炎症性疾病中的作用需要鉴定微生物效应分子。我们通过整合配对宏基因组学、粪便和血浆代谢组学以及培养组学,建立了一种将疾病相关微生物与微生物代谢物联系起来的方法。我们在预测标准化结肠炎治疗 (PROTECT) 儿科初始队列反应中确定了与疾病活动性、炎症和溃疡性结肠炎 (UC) 临床病程相关的宿主-微生物相互作用。在严重疾病中,代谢变化包括粪便中二肽和牛磺结合胆汁酸 (BA) 增加以及氨基酸结合胆汁酸 (BA) 减少,而血浆多胺(N-乙酰腐胺和 N1-乙酰亚精胺)增加。使用患者样本和小韦荣球菌作为模型,我们发现了硝酸盐和乳酸依赖性代谢途径,通过实验将小韦荣球菌的扩张与免疫调节色氨酸代谢物的产生联系起来。此外,V. parvula通过 xdhA 黄嘌呤脱氢酶代谢免疫抑制硫嘌呤药物,可能会损害治疗反应。我们的研究结果表明,微生物组有助于与疾病相关的代谢物变化,强调了这些相互作用在疾病病理学和治疗中的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号