当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Complexation by Particulate Organic Matter Alters Iron Redox Behavior
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-01-11 , DOI: 10.1021/acsearthspacechem.3c00288 Prachi Joshi 1 , Laurel K. ThomasArrigo 2, 3 , Dennis Sawwa 1 , Lea Sauter 1 , Andreas Kappler 1, 4
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-01-11 , DOI: 10.1021/acsearthspacechem.3c00288 Prachi Joshi 1 , Laurel K. ThomasArrigo 2, 3 , Dennis Sawwa 1 , Lea Sauter 1 , Andreas Kappler 1, 4
Affiliation
|
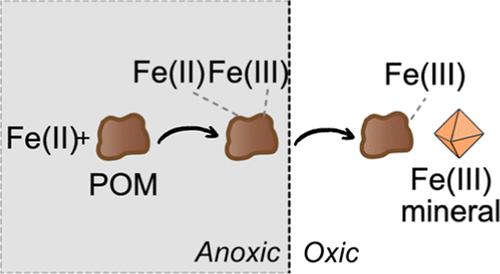
The role of iron (Fe) in environmental redox processes, such as microbial respiration and pollutant turnover, is influenced by its association with organic matter, particularly in redox-dynamic systems such as wetlands. While the association between Fe and dissolved organic matter (DOM) has been studied extensively, the association between Fe and particulate organic matter (POM), which differs in size and composition from DOM, is poorly understood. In this work, we investigated the complexation of aqueous Fe(II) by mineral-free POM over a full redox cycle using wet chemical and spectroscopic (X-ray absorption and Mössbauer spectroscopy) techniques. The mass of Fe(II) complexed by POM under anoxic conditions ranged from 18.9 ± 1.2 mg Fe·g–1 POM at pH 4.5 to 37.6 ± 1.5 mg Fe·g–1 POM at pH 7. Part of the complexed Fe(II) was oxidized to Fe(III) (21–46%) under anoxic conditions, indicating that complexation by POM altered the Fe(II) redox stability. We then exposed POM-complexed Fe to O2 at pH 5.5 and 7 to simulate oxidizing conditions. Upon exposure to aqueous O2, the complexed Fe(II) was rapidly and completely oxidized at pH 5.5 and pH 7, faster than uncomplexed Fe(II), suggesting that complexation by POM promoted abiotic Fe(II) oxidation. The resulting Fe(III)-POM consisted of Fe(III)-organic phases and poorly crystalline Fe(III) (oxyhydr)oxides. The results of this work demonstrate that POM acts as a major complexant of Fe and alters Fe redox behavior, thereby affecting the role of Fe in environmental redox processes.
中文翻译:

颗粒有机物质的络合改变了铁的氧化还原行为
铁 (Fe) 在环境氧化还原过程(例如微生物呼吸和污染物周转)中的作用受到其与有机物的关联的影响,特别是在湿地等氧化还原动力系统中。虽然 Fe 和溶解有机物 (DOM) 之间的关联已得到广泛研究,但人们对 Fe 和颗粒有机物 (POM) 之间的关联知之甚少,POM 的尺寸和成分与 DOM 不同。在这项工作中,我们使用湿化学和光谱(X 射线吸收和穆斯堡尔光谱)技术研究了在整个氧化还原循环中无矿物 POM 与水性 Fe(II) 的络合。在缺氧条件下,POM 络合的 Fe(II) 质量范围为 pH 4.5 时的 18.9 ± 1.2 mg Fe·g –1 POM 至 pH 7 时的 37.6 ± 1.5 mg Fe·g –1 POM。 )在缺氧条件下被氧化为 Fe(III) (21–46%),表明 POM 的络合作用改变了 Fe(II) 的氧化还原稳定性。然后,我们将 POM 络合的 Fe 暴露于pH 5.5 和 7 的O 2中以模拟氧化条件。暴露于 O 2水溶液后,络合的 Fe(II) 在 pH 5.5 和 pH 7 下迅速且完全氧化,比未络合的 Fe(II) 更快,表明 POM 络合促进了非生物 Fe(II) 氧化。所得 Fe(III)-POM 由 Fe(III) 有机相和结晶不良的 Fe(III)(羟基)氧化物组成。这项工作的结果表明,POM 作为 Fe 的主要络合剂并改变 Fe 的氧化还原行为,从而影响 Fe 在环境氧化还原过程中的作用。
更新日期:2024-01-11
中文翻译:

颗粒有机物质的络合改变了铁的氧化还原行为
铁 (Fe) 在环境氧化还原过程(例如微生物呼吸和污染物周转)中的作用受到其与有机物的关联的影响,特别是在湿地等氧化还原动力系统中。虽然 Fe 和溶解有机物 (DOM) 之间的关联已得到广泛研究,但人们对 Fe 和颗粒有机物 (POM) 之间的关联知之甚少,POM 的尺寸和成分与 DOM 不同。在这项工作中,我们使用湿化学和光谱(X 射线吸收和穆斯堡尔光谱)技术研究了在整个氧化还原循环中无矿物 POM 与水性 Fe(II) 的络合。在缺氧条件下,POM 络合的 Fe(II) 质量范围为 pH 4.5 时的 18.9 ± 1.2 mg Fe·g –1 POM 至 pH 7 时的 37.6 ± 1.5 mg Fe·g –1 POM。 )在缺氧条件下被氧化为 Fe(III) (21–46%),表明 POM 的络合作用改变了 Fe(II) 的氧化还原稳定性。然后,我们将 POM 络合的 Fe 暴露于pH 5.5 和 7 的O 2中以模拟氧化条件。暴露于 O 2水溶液后,络合的 Fe(II) 在 pH 5.5 和 pH 7 下迅速且完全氧化,比未络合的 Fe(II) 更快,表明 POM 络合促进了非生物 Fe(II) 氧化。所得 Fe(III)-POM 由 Fe(III) 有机相和结晶不良的 Fe(III)(羟基)氧化物组成。这项工作的结果表明,POM 作为 Fe 的主要络合剂并改变 Fe 的氧化还原行为,从而影响 Fe 在环境氧化还原过程中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号