当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of carbonate on ferrihydrite transformation in alkaline media
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2024-01-03 , DOI: 10.1039/d3em00469d Ying Li 1, 2 , Chaoqun Zhang 3 , Meijun Yang 3 , Jing Liu 4 , Hongping He 3 , Yibing Ma 1 , Yuji Arai 2
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2024-01-03 , DOI: 10.1039/d3em00469d Ying Li 1, 2 , Chaoqun Zhang 3 , Meijun Yang 3 , Jing Liu 4 , Hongping He 3 , Yibing Ma 1 , Yuji Arai 2
Affiliation
|
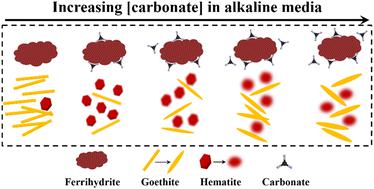
Alkaline media widely exist in natural and engineered systems such as semiarid/arid areas, radioactive waste sites, and mine tailings. In these settings, the commonly occurring iron (oxyhydr)oxides differed in their ability to influence the fate of nutrients and contaminants. Due to the substantially increased atmospheric carbon dioxide (CO2) concentration, carbonate stands to increase in these media. However, how increasing carbonate affects the transformation of poorly crystalline iron (oxyhydr)oxides (e.g., two-line ferrihydrite) under alkaline conditions still remains unclear. Here, kinetics of ferrihydrite transformation were evaluated at pH ∼10 as a function of [carbonate] = 0–286 mM using synchrotron-based X-ray and vibrational spectroscopic techniques. The results showed that carbonate slowed down ferrihydrite transformation slightly and suppressed goethite formation, but promoted hematite formation regardless of its concentration. At low carbonate concentration (11.42 mM), the effect of carbonate on product formation was obvious due to the weak inner-sphere complex; however, at high carbonate concentration (80–286 mM), the effect was retarded because of the adsorption equilibrium of carbonate as well as the initial carbonate adsorption followed by desorption. Moreover, carbonate modified the morphology of hematite from rhombic to ellipsoidal to honeycomb and goethite from rod-like to needle-like to spindle-like due to the inner-sphere adsorption–desorption of carbonate and adsorption of hydroxyl ions on reactive sites of iron (oxyhydr)oxides in alkaline media. The results suggest that the concurrently increasing carbonate with enhanced atmospheric CO2 could control the transformation and occurrence of iron (oxyhydr)oxides in natural and engineered environments and have important implications for the biogeochemical cycles of iron and carbon.
中文翻译:

碳酸盐对碱性介质中水铁矿转化的影响
碱性介质广泛存在于自然和工程系统中,例如半干旱/干旱地区、放射性废物场和尾矿。在这些环境中,常见的铁(羟基)氧化物影响营养物和污染物的能力不同。由于大气二氧化碳 (CO 2 ) 浓度大幅增加,这些介质中的碳酸盐也会增加。然而,增加碳酸盐如何影响碱性条件下结晶不良的铁(羟基)氧化物(例如,双线水铁矿)的转化仍不清楚。在这里,使用基于同步加速器的 X 射线和振动光谱技术,在 pH ∼10 下评估了水铁矿转化动力学,作为 [碳酸盐] = 0–286 mM 的函数。结果表明,碳酸盐稍微减缓了水铁矿的转变并抑制了针铁矿的形成,但无论其浓度如何,都会促进赤铁矿的形成。在低碳酸盐浓度(11.42 mM)下,由于内球复合物较弱,碳酸盐对产物形成的影响明显;然而,在高碳酸盐浓度(80-286 mM)下,由于碳酸盐的吸附平衡以及碳酸盐初始吸附和解吸,效果会受到阻碍。此外,由于碳酸盐的内球吸附-解吸和铁反应位点上羟基离子的吸附,碳酸盐改变了赤铁矿的形态从菱形到椭圆形再到蜂窝状,针铁矿的形态从棒状到针状再到纺锤状。碱性介质中的羟基氧化物。结果表明,碳酸盐的同时增加和大气CO 2的增加可以控制自然和工程环境中铁(羟基)氧化物的转化和出现,并对铁和碳的生物地球化学循环具有重要意义。
更新日期:2024-01-03
中文翻译:

碳酸盐对碱性介质中水铁矿转化的影响
碱性介质广泛存在于自然和工程系统中,例如半干旱/干旱地区、放射性废物场和尾矿。在这些环境中,常见的铁(羟基)氧化物影响营养物和污染物的能力不同。由于大气二氧化碳 (CO 2 ) 浓度大幅增加,这些介质中的碳酸盐也会增加。然而,增加碳酸盐如何影响碱性条件下结晶不良的铁(羟基)氧化物(例如,双线水铁矿)的转化仍不清楚。在这里,使用基于同步加速器的 X 射线和振动光谱技术,在 pH ∼10 下评估了水铁矿转化动力学,作为 [碳酸盐] = 0–286 mM 的函数。结果表明,碳酸盐稍微减缓了水铁矿的转变并抑制了针铁矿的形成,但无论其浓度如何,都会促进赤铁矿的形成。在低碳酸盐浓度(11.42 mM)下,由于内球复合物较弱,碳酸盐对产物形成的影响明显;然而,在高碳酸盐浓度(80-286 mM)下,由于碳酸盐的吸附平衡以及碳酸盐初始吸附和解吸,效果会受到阻碍。此外,由于碳酸盐的内球吸附-解吸和铁反应位点上羟基离子的吸附,碳酸盐改变了赤铁矿的形态从菱形到椭圆形再到蜂窝状,针铁矿的形态从棒状到针状再到纺锤状。碱性介质中的羟基氧化物。结果表明,碳酸盐的同时增加和大气CO 2的增加可以控制自然和工程环境中铁(羟基)氧化物的转化和出现,并对铁和碳的生物地球化学循环具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号