当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metabolic switch regulates lineage plasticity and induces synthetic lethality in triple-negative breast cancer
Cell Metabolism ( IF 29.0 ) Pub Date : 2024-01-02 , DOI: 10.1016/j.cmet.2023.12.003 Yingsheng Zhang , Meng-Ju Wu , Wan-Chi Lu , Yi-Chuan Li , Chun Ju Chang , Jer-Yen Yang
Cell Metabolism ( IF 29.0 ) Pub Date : 2024-01-02 , DOI: 10.1016/j.cmet.2023.12.003 Yingsheng Zhang , Meng-Ju Wu , Wan-Chi Lu , Yi-Chuan Li , Chun Ju Chang , Jer-Yen Yang
|
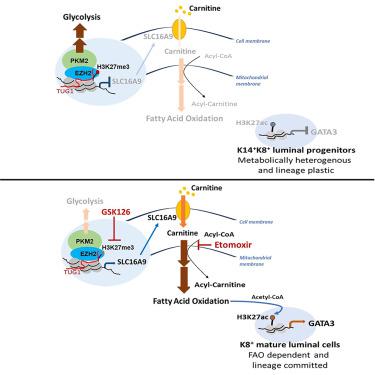
Metabolic reprogramming is key for cancer development, yet the mechanism that sustains triple-negative breast cancer (TNBC) cell growth despite deficient pyruvate kinase M2 (PKM2) and tumor glycolysis remains to be determined. Here, we find that deficiency in tumor glycolysis activates a metabolic switch from glycolysis to fatty acid β-oxidation (FAO) to fuel TNBC growth. We show that, in TNBC cells, PKM2 directly interacts with histone methyltransferase EZH2 to coordinately mediate epigenetic silencing of a carnitine transporter, . Inhibition of PKM2 leads to impaired EZH2 recruitment to , and in turn de-represses SLC16A9 expression to increase intracellular carnitine influx, programming TNBC cells to an FAO-dependent and luminal-like cell state. Together, these findings reveal a new metabolic switch that drives TNBC from a metabolically heterogeneous-lineage plastic cell state to an FAO-dependent-lineage committed cell state, where dual targeting of EZH2 and FAO induces potent synthetic lethality in TNBC.
中文翻译:

代谢开关调节谱系可塑性并诱导三阴性乳腺癌的综合致死性
代谢重编程是癌症发展的关键,但尽管丙酮酸激酶 M2 (PKM2) 和肿瘤糖酵解缺陷,但维持三阴性乳腺癌 (TNBC) 细胞生长的机制仍有待确定。在这里,我们发现肿瘤糖酵解缺陷会激活从糖酵解到脂肪酸β-氧化(FAO)的代谢转换,以促进 TNBC 的生长。我们发现,在 TNBC 细胞中,PKM2 直接与组蛋白甲基转移酶 EZH2 相互作用,协调介导肉碱转运蛋白的表观遗传沉默。抑制 PKM2 会导致 EZH2 募集受损,进而解除 SLC16A9 表达的抑制,从而增加细胞内肉碱流入,将 TNBC 细胞编程为依赖于 FAO 的管腔样细胞状态。总之,这些发现揭示了一种新的代谢转换,可驱动 TNBC 从代谢异质谱系塑性细胞状态转变为依赖于 FAO 的谱系定型细胞状态,其中 EZH2 和 FAO 的双重靶向可在 TNBC 中诱导有效的合成致死性。
更新日期:2024-01-02
中文翻译:

代谢开关调节谱系可塑性并诱导三阴性乳腺癌的综合致死性
代谢重编程是癌症发展的关键,但尽管丙酮酸激酶 M2 (PKM2) 和肿瘤糖酵解缺陷,但维持三阴性乳腺癌 (TNBC) 细胞生长的机制仍有待确定。在这里,我们发现肿瘤糖酵解缺陷会激活从糖酵解到脂肪酸β-氧化(FAO)的代谢转换,以促进 TNBC 的生长。我们发现,在 TNBC 细胞中,PKM2 直接与组蛋白甲基转移酶 EZH2 相互作用,协调介导肉碱转运蛋白的表观遗传沉默。抑制 PKM2 会导致 EZH2 募集受损,进而解除 SLC16A9 表达的抑制,从而增加细胞内肉碱流入,将 TNBC 细胞编程为依赖于 FAO 的管腔样细胞状态。总之,这些发现揭示了一种新的代谢转换,可驱动 TNBC 从代谢异质谱系塑性细胞状态转变为依赖于 FAO 的谱系定型细胞状态,其中 EZH2 和 FAO 的双重靶向可在 TNBC 中诱导有效的合成致死性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号