Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.jfluchem.2023.110237 Caicai He , Swastik Karmakar , Dandan Wei , Wei Zhao , Xiaolong Zhang , Xihe Bi
|
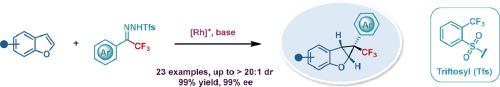
An asymmetric dearomative cyclopropanation of benzofuran has been accomplished by a novel catalytic method that relies on using trifluoromethyl N-triftosylhydrazones as carbene sources in the presence of a chiral rhodium catalyst. This reaction produces chiral trifluoromethyl-tethered 2,3-disubstituted benzofuran cyclopropane, which carries versatile pharmacophores 2,3-dihydrobenzofuran and trifluoromethyl-substituted quaternary carbon centers. Notably, this process offers distinct advantages over other existing approaches due to being step-economic and eliminating green-gas N2 as a harmless coproduct. DFT calculations explain the reason behind the high enantioselectivity during this cyclopropanation reaction.
中文翻译:

Rh催化苯并呋喃与三氟甲基N-三甲苯基腙的不对称环丙烷化反应
苯并呋喃的不对称脱芳环丙烷化反应是通过一种新颖的催化方法完成的,该方法依赖于在手性铑催化剂存在下使用三氟甲基N-三甲苯基腙作为卡宾源。该反应产生手性三氟甲基连接的2,3-二取代苯并呋喃环丙烷,其带有通用药效团2,3-二氢苯并呋喃和三氟甲基取代的季碳中心。值得注意的是,由于步骤经济且消除了作为无害副产品的绿色气体 N 2 ,因此该工艺比其他现有方法具有明显的优势。DFT 计算解释了环丙烷化反应中高对映选择性背后的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号