当前位置:
X-MOL 学术
›
Am. J. Kidney Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anti–Phospholipase A2 Receptor 1 and Anti–Cysteine Rich Antibodies, Domain Recognition and Rituximab Efficacy in Membranous Nephropathy: A Prospective Cohort Study
American Journal of Kidney Diseases ( IF 13.2 ) Pub Date : 2023-12-25 , DOI: 10.1053/j.ajkd.2023.10.013 Piero Ruggenenti , Linda Reinhard , Barbara Ruggiero , Annalisa Perna , Luca Perico , Tobia Peracchi , Diego Fidone , Alessia Gennarini , Ariela Benigni , Monica Cortinovis , Elion Hoxha , Giuseppe Remuzzi
American Journal of Kidney Diseases ( IF 13.2 ) Pub Date : 2023-12-25 , DOI: 10.1053/j.ajkd.2023.10.013 Piero Ruggenenti , Linda Reinhard , Barbara Ruggiero , Annalisa Perna , Luca Perico , Tobia Peracchi , Diego Fidone , Alessia Gennarini , Ariela Benigni , Monica Cortinovis , Elion Hoxha , Giuseppe Remuzzi
|
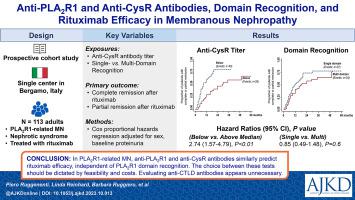
Proteinuria and anti–phospholipase A receptor 1 (anti-PLAR1) antibody titers are associated with primary membranous nephropathy (MN) outcomes. We evaluated the association of antibodies against the cysteine-rich (CysR) and C-type lectin 1, 7, and 8 (CTLD1, CTLD7, and CTLD8) domains of PLAR1 with MN outcomes. Prospective cohort study. One-hundred-thirteen consecutive, consenting patients referred to the Nephology Unit of the Azienda-Socio-Sanitaria-Territoriale (ASST) Papa Giovanni XXIII (Bergamo, Italy) with PLAR1-related, biopsy-proven MN whose persistent nephrotic syndrome (NS) was managed conservatively for>6 months and were monitored with serial evaluations of proteinuria, autoantibodies (by enzyme-linked immunosorbent assay), and clinical outcomes. Rituximab. Complete (proteinuria<0.3g/24h) or partial (proteinuria≥0.3g/24h and<3.0g/24h with>50% reduction vs basal) NS remission. Univariable and multivariable Cox regression analyses. All patients had anti-CysR antibodies; 62 (54.9%) were multidomain recognizers. Anti-PLAR1 and anti-CysR antibody titers were strongly correlated at baseline (<0.001, =0.934), 6 months (<0.001, =0.964), and 12 months (<0.001, =0.944). During a median follow-up of 37.1 (IQR, 20.3-56.9) months, 71 patients (62.8%) achieved either complete or partial remission of their NS. Lower baseline anti-PLAR1 (HR, 0.997 [95% CI, 0.996-0.999], =0.002) and anti-CysR [HR, 0.996 [95% CI, 0.993-0.998], =0.001) titers were associated with a higher probability of remission, along with female sex, lower proteinuria, and lower serum creatinine levels (<0.05 for all comparisons). Anti-CTLD antibodies were not associated with outcomes. At 6 and 12 months, compared to baseline, anti-PLAR1 and anti-CysR antibody titers decreased more in patients progressing to partial or complete remission than in those without remission (<0.05 for all comparisons). Observational design. In PLAR1-related MN, anti-PLAR1 and anti-CysR antibodies similarly predict rituximab efficacy independent of PLAR1 domain recognition. The choice between these tests should be dictated by feasibility and costs. Evaluating anti-CTLD antibodies appears unnecessary. Primary membranous nephropathy (MN), a leading cause of nephrotic syndrome (NS) in adults, is an autoimmune disease caused by autoantibodies binding to the podocyte antigen phospholipase A2 receptor 1 (PLAR1). We assessed whether the effects of anti-CD20 cytolytic therapy with the monoclonal antibody rituximab are associated with detection rates and levels of anti-PLAR1 antibodies and antibodies against PLAR1 domains such as cysteine-rich (CysR), and C-type lectin 1, 7, and 8 (CTLD1, 7, and 8), in patients with PLAR1-related MN and persistent NS. The probability of rituximab-induced complete or partial NS remission was associated with baseline anti-PLAR1 and anti-CysR antibody titers, but not with anti-CTLD1, 7 and 8 antibodies or multidomain recognition. Integrated evaluation of anti-PLAR1 or anti-CysR antibodies with proteinuria and kidney function may play a role in monitoring the effects of rituximab in patients with PLAR1-related NS and MN.
中文翻译:

抗磷脂酶 A2 受体 1 和抗半胱氨酸富集抗体、结构域识别和利妥昔单抗在膜性肾病中的疗效:一项前瞻性队列研究
蛋白尿和抗磷脂酶 A 受体 1 (抗 PLAR1) 抗体滴度与原发性膜性肾病 (MN) 结局相关。我们评估了 PLAR1 富含半胱氨酸 (CysR) 和 C 型凝集素 1、7 和 8(CTLD1、CTLD7 和 CTLD8)结构域的抗体与 MN 结果之间的关联。前瞻性队列研究。 113 名连续同意的患者被转诊至 Azienda-Socio-Sanitaria-Territoriale (ASST) Papa Giovanni XXIII(意大利贝加莫)肾病科,患有与 PLAR1 相关、经活检证实的 MN,其持续性肾病综合征 (NS)保守治疗超过 6 个月,并通过蛋白尿、自身抗体(通过酶联免疫吸附测定)和临床结果的系列评估进行监测。利妥昔单抗。完全(蛋白尿<0.3g/24h)或部分(蛋白尿≥0.3g/24h和<3.0g/24h,与基础相比减少>50%)NS缓解。单变量和多变量 Cox 回归分析。所有患者均有抗CysR抗体; 62 个(54.9%)是多域识别器。抗PLAR1和抗CysR抗体滴度在基线(<0.001,=0.934)、6个月(<0.001,=0.964)和12个月(<0.001,=0.944)时密切相关。在中位随访 37.1(IQR,20.3-56.9)个月期间,71 名患者 (62.8%) 的 NS 达到完全或部分缓解。较低基线抗 PLAR1(HR,0.997 [95% CI,0.996-0.999],=0.002)和抗 CysR [HR,0.996 [95% CI,0.993-0.998],=0.001)滴度与较高概率相关缓解,以及女性性别、较低的蛋白尿和较低的血清肌酐水平(所有比较<0.05)。抗 CTLD 抗体与结果无关。在第 6 个月和第 12 个月时,与基线相比,进展至部分或完全缓解的患者的抗 PLAR1 和抗 CysR 抗体滴度比未缓解的患者下降更多(所有比较 <0.05)。观察设计。在 PLAR1 相关的 MN 中,抗 PLAR1 和抗 CysR 抗体类似地预测利妥昔单抗疗效,与 PLAR1 结构域识别无关。这些测试之间的选择应根据可行性和成本来决定。评估抗 CTLD 抗体似乎没有必要。原发性膜性肾病 (MN) 是成人肾病综合征 (NS) 的主要原因,是一种由自身抗体与足细胞抗原磷脂酶 A2 受体 1 (PLAR1) 结合引起的自身免疫性疾病。我们评估了单克隆抗体利妥昔单抗抗 CD20 细胞溶解疗法的效果是否与抗 PLAR1 抗体和抗 PLAR1 结构域(例如富含半胱氨酸 (CysR) 和 C 型凝集素 1, 7)的抗体的检出率和水平相关。 、和 8(CTLD1、7 和 8),在 PLAR1 相关的 MN 和持续性 NS 患者中。利妥昔单抗诱导的完全或部分 NS 缓解的概率与基线抗 PLAR1 和抗 CysR 抗体滴度相关,但与抗 CTLD1、7 和 8 抗体或多域识别无关。抗PLAR1或抗CysR抗体与蛋白尿和肾功能的综合评估可能在监测利妥昔单抗对PLAR1相关NS和MN患者的疗效中发挥作用。
更新日期:2023-12-25
中文翻译:

抗磷脂酶 A2 受体 1 和抗半胱氨酸富集抗体、结构域识别和利妥昔单抗在膜性肾病中的疗效:一项前瞻性队列研究
蛋白尿和抗磷脂酶 A 受体 1 (抗 PLAR1) 抗体滴度与原发性膜性肾病 (MN) 结局相关。我们评估了 PLAR1 富含半胱氨酸 (CysR) 和 C 型凝集素 1、7 和 8(CTLD1、CTLD7 和 CTLD8)结构域的抗体与 MN 结果之间的关联。前瞻性队列研究。 113 名连续同意的患者被转诊至 Azienda-Socio-Sanitaria-Territoriale (ASST) Papa Giovanni XXIII(意大利贝加莫)肾病科,患有与 PLAR1 相关、经活检证实的 MN,其持续性肾病综合征 (NS)保守治疗超过 6 个月,并通过蛋白尿、自身抗体(通过酶联免疫吸附测定)和临床结果的系列评估进行监测。利妥昔单抗。完全(蛋白尿<0.3g/24h)或部分(蛋白尿≥0.3g/24h和<3.0g/24h,与基础相比减少>50%)NS缓解。单变量和多变量 Cox 回归分析。所有患者均有抗CysR抗体; 62 个(54.9%)是多域识别器。抗PLAR1和抗CysR抗体滴度在基线(<0.001,=0.934)、6个月(<0.001,=0.964)和12个月(<0.001,=0.944)时密切相关。在中位随访 37.1(IQR,20.3-56.9)个月期间,71 名患者 (62.8%) 的 NS 达到完全或部分缓解。较低基线抗 PLAR1(HR,0.997 [95% CI,0.996-0.999],=0.002)和抗 CysR [HR,0.996 [95% CI,0.993-0.998],=0.001)滴度与较高概率相关缓解,以及女性性别、较低的蛋白尿和较低的血清肌酐水平(所有比较<0.05)。抗 CTLD 抗体与结果无关。在第 6 个月和第 12 个月时,与基线相比,进展至部分或完全缓解的患者的抗 PLAR1 和抗 CysR 抗体滴度比未缓解的患者下降更多(所有比较 <0.05)。观察设计。在 PLAR1 相关的 MN 中,抗 PLAR1 和抗 CysR 抗体类似地预测利妥昔单抗疗效,与 PLAR1 结构域识别无关。这些测试之间的选择应根据可行性和成本来决定。评估抗 CTLD 抗体似乎没有必要。原发性膜性肾病 (MN) 是成人肾病综合征 (NS) 的主要原因,是一种由自身抗体与足细胞抗原磷脂酶 A2 受体 1 (PLAR1) 结合引起的自身免疫性疾病。我们评估了单克隆抗体利妥昔单抗抗 CD20 细胞溶解疗法的效果是否与抗 PLAR1 抗体和抗 PLAR1 结构域(例如富含半胱氨酸 (CysR) 和 C 型凝集素 1, 7)的抗体的检出率和水平相关。 、和 8(CTLD1、7 和 8),在 PLAR1 相关的 MN 和持续性 NS 患者中。利妥昔单抗诱导的完全或部分 NS 缓解的概率与基线抗 PLAR1 和抗 CysR 抗体滴度相关,但与抗 CTLD1、7 和 8 抗体或多域识别无关。抗PLAR1或抗CysR抗体与蛋白尿和肾功能的综合评估可能在监测利妥昔单抗对PLAR1相关NS和MN患者的疗效中发挥作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号