The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2023-12-20 , DOI: 10.1016/j.supflu.2023.106149 Guoxing Li , Shaoguang Zhang , Mingbo Niu , Chuang Yang
|
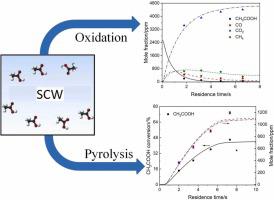
The oxidation and hydrolysis of 0.11–0.14 M acetic acid in supercritical water (SCW) have been investigated at temperatures of 773–873 K and a pressure of 25.0 MPa in a flow reactor. CO and CO2 were the main products at oxygen-rich conditions. For hydrolysis experiments, CH4 and CO2 were produced in nearly equal amounts while the yield of CO was negligibly small. A detailed chemical kinetic model was developed by revising the kinetic parameters of key elementary reactions. The model performance was evaluated by comparison to the present data as well as data from literature. The model predicted the kinetic behavior of acetic acid oxidation fairly well, despite of slight underprediction of CO and overprediction of CH4. It also reproduced satisfactorily the species concentrations during acetic acid hydrolysis at high temperatures. Based on the model, kinetic analyses were further conducted to identify important elementary steps and to infer reaction mechanisms.
中文翻译:

超临界水中乙酸氧化和水解的实验和动力学模型研究
在流动反应器中,在 773-873 K 的温度和 25.0 MPa 的压力下,研究了超临界水 (SCW) 中 0.11-0.14 M 乙酸的氧化和水解。CO和CO 2是富氧条件下的主要产物。对于水解实验,CH 4和CO 2的产生量几乎相等,而CO 的产量小得可以忽略不计。通过修改关键基元反应的动力学参数,建立了详细的化学动力学模型。通过与现有数据以及文献数据进行比较来评估模型性能。该模型相当好地预测了乙酸氧化的动力学行为,尽管对 CO 的预测略有偏低,对 CH 4的预测偏高。它还令人满意地再现了高温乙酸水解过程中的物质浓度。基于该模型,进一步进行动力学分析,以确定重要的基本步骤并推断反应机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号