Neuropharmacology ( IF 4.7 ) Pub Date : 2023-12-16 , DOI: 10.1016/j.neuropharm.2023.109817 Kiyofumi Yamamoto , Satoshi Kosukegawa , Masayuki Kobayashi
|
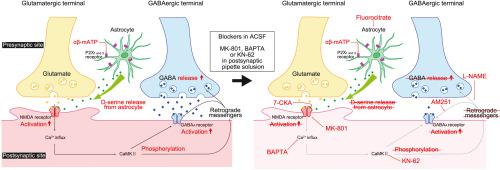
Adenosine triphosphate (ATP) changes the efficacy of synaptic transmission. Despite recent progress in terms of the roles of purinergic receptors in cerebrocortical excitatory synaptic transmission, their contribution to inhibitory synaptic transmission is unknown. To elucidate the effects of α,β-methylene ATP (αβ-mATP), a selective agonist of P2X receptors (P2XRs), on inhibitory synaptic transmission in the insular cortex (IC), we performed whole-cell patch-clamp recording from IC pyramidal neurons (PNs) and fast-spiking neurons (FSNs) in either sex of VGAT-Venus transgenic rats. αβ-mATP increased the amplitude of miniature IPSCs (mIPSCs) under conditions in which NMDA receptors (NMDARs) are recruitable. αβ-mATP-induced facilitation of mIPSCs was sustained even after the washout of αβ-mATP, which was blocked by preincubation with fluorocitrate. The preapplication of NF023 (a P2X1 receptor antagonist) or AF-353 (a P2X3 receptor antagonist) blocked αβ-mATP-induced mIPSC facilitation. Intracellular application of the NMDAR antagonist MK801 blocked the facilitation. d-serine, which is an intrinsic agonist of NMDARs, mimicked αβ-mATP-induced mIPSC facilitation. The intracellular application of BAPTA a Ca2+ chelator, or the bath application of KN-62, a CaMKII inhibitor, blocked αβ-mATP-induced mIPSC facilitation, thus indicating that mIPSC facilitation by αβ-mATP required postsynaptic [Ca2+]i elevation through NMDAR activation. Paired whole-cell patch-clamp recordings from FSNs and PNs demonstrated that αβ-mATP increased the amplitude of unitary IPSCs without changing the paired-pulse ratio. These results suggest that αβ-mATP-induced IPSC facilitation is mediated by postsynaptic NMDAR activations through d-serine released from astrocytes. Subsequent [Ca2+]i increase and postsynaptic CaMKII activation may release retrograde messengers that upregulate GABA release from presynaptic inhibitory neurons, including FSNs. (250/250 words)
中文翻译:

P2X 受体和突触后 NMDA 受体介导的大鼠岛叶皮质抑制性突触的持久促进
三磷酸腺苷 (ATP) 改变突触传递的功效。尽管最近在嘌呤能受体在大脑皮质兴奋性突触传递中的作用方面取得了进展,但它们对抑制性突触传递的贡献尚不清楚。为了阐明 α,β-亚甲基 ATP (αβ-mATP)(一种 P2X 受体 (P2XR) 的选择性激动剂)对岛叶皮质(IC)抑制性突触传递的影响,我们对 IC 进行了全细胞膜片钳记录VGAT-Venus 转基因大鼠任一性别的锥体神经元 (PN) 和快速尖峰神经元 (FSN) 。在 NMDA 受体 (NMDAR) 可招募的条件下,αβ-mATP 增加了微型 IPSC (mIPSC) 的振幅。即使在洗掉 αβ-mATP 后,αβ-mATP 诱导的 mIPSC 促进作用仍然持续,而氟柠檬酸盐预孵育可阻断该作用。预先应用 NF023(一种 P2X 1受体拮抗剂)或 AF-353(一种 P2X3受体拮抗剂)可阻断 αβ-mATP 诱导的 mIPSC 促进。细胞内应用 NMDAR 拮抗剂MK801 阻断了这种促进作用。d-丝氨酸是 NMDAR 的内在激动剂,模拟 αβ-mATP 诱导的 mIPSC 促进作用。细胞内应用 BAPTA(一种 Ca2+螯合剂)或浴应用 KN-62(一种CaMKII 抑制剂)可阻断 αβ-mATP 诱导的 mIPSC 促进,从而表明 αβ-mATP 促进 mIPSC 需要突触后 [Ca2+]i通过 NMDAR 激活升高。FSN 和 PN 的配对全细胞膜片钳记录表明,αβ-mATP 增加了单一 IPSC 的振幅,而不改变配对脉冲比。这些结果表明,αβ-mATP 诱导的 IPSC 促进是通过d-丝氨酸,由突触后 NMDAR 激活介导的。随后的 [Ca2+]i增加和突触后 CaMKII 激活可能会释放逆行信使,从而上调突触前抑制神经元(包括 FSN)的 GABA 释放。(250/250字)



























 京公网安备 11010802027423号
京公网安备 11010802027423号