Cellular Immunology ( IF 4.3 ) Pub Date : 2023-12-10 , DOI: 10.1016/j.cellimm.2023.104795 Fernanda Agostini Rocha , Caio Raony Farina Silveira , Ancély Ferreira dos Santos , Ana Carolina Buzzo Stefanini , Nelson Hamerschlak , Luciana Cavalheiro Marti
|
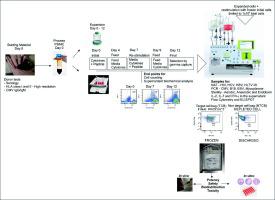
At present, recipients of allogeneic hematopoietic stem-cells are still suffering from recurrent infections after transplantation. Infusion of virus-specific T cells (VST) post-transplant reportedly fights several viruses without increasing the risk of de novo graft-versus-host disease. This study targeted cytomegalovirus (CMV) for the development of an innovative approach for generating a very specific VST product following Good Manufacturing Practices (GMP) guidelines.
We used a sterile disposable compartment named the Leukoreduction System Chamber (LRS-chamber) from the apheresis platelet donation kit as the starting material, which has demonstrated high levels of T cells. Using a combination of IL-2 and IL-7 we could improve expansion of CMV-specific T cells. Moreover, by developing and establishing a new product protocol, we were able to stimulate VST proliferation and favors T cell effector memory profile. The expanded VST were enriched in a closed automated system, creating a highly pure anti-CMV product, which was pre-clinically tested for specificity in vitro and for persistence, biodistribution, and toxicity in vivo using NOD scid mice. Our results demonstrated very specific VST, able to secrete high amounts of interferon only in the presence of cells infected by the human CMV strain (AD169), and innocuous to cells partially HLA compatible without viral infection.
中文翻译:

开发用于过继性 T 细胞治疗的高细胞毒性、临床级病毒特异性 T 细胞产品
目前,异体造血干细胞的受者仍面临移植后反复感染的问题。据报道,移植后输注病毒特异性 T 细胞 (VST) 可以对抗多种病毒,而不会增加新发移植物抗宿主病的风险。这项研究以巨细胞病毒 (CMV) 为目标,开发一种创新方法,按照良好生产规范 (GMP) 指南生成非常具体的 VST 产品。
我们使用单采血小板捐赠试剂盒中名为白细胞减少系统室(LRS-室)的无菌一次性隔室作为起始材料,该隔室已显示出高水平的 T 细胞。使用 IL-2 和 IL-7 的组合,我们可以改善 CMV 特异性 T 细胞的扩增。此外,通过开发和建立新的产品方案,我们能够刺激 VST 增殖并有利于 T 细胞效应记忆特征。扩展的 VST 在封闭的自动化系统中富集,形成高纯度的抗 CMV 产品,并使用 NOD scid 小鼠对该产品进行了临床前体外特异性测试以及体内持久性、生物分布和毒性测试。我们的结果表明,VST 具有非常特异性,仅在被人类 CMV 毒株 (AD169) 感染的细胞存在时才能够分泌大量干扰素,并且对部分 HLA 相容且无病毒感染的细胞无害。



























 京公网安备 11010802027423号
京公网安备 11010802027423号