当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural mechanism of Escherichia coli cyanase
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2023-11-16 , DOI: 10.1107/s2059798323009609 Jihan Kim 1 , Youngchang Kim 2 , Jaehyun Park 3 , Ki Hyun Nam 1 , Yunje Cho 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2023-11-16 , DOI: 10.1107/s2059798323009609 Jihan Kim 1 , Youngchang Kim 2 , Jaehyun Park 3 , Ki Hyun Nam 1 , Yunje Cho 1
Affiliation
|
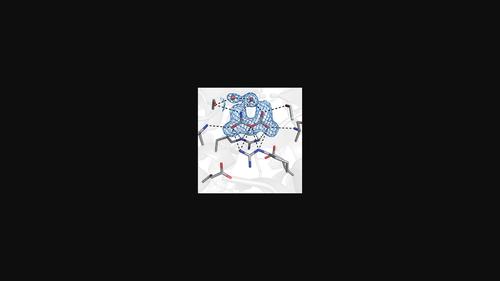
Cyanase plays a vital role in the detoxification of cyanate and supplies a continuous nitrogen source for soil microbes by converting cyanate to ammonia and carbon dioxide in a bicarbonate-dependent reaction. The structures of cyanase complexed with dianion inhibitors, in conjunction with biochemical studies, suggest putative binding sites for substrates. However, the substrate-recognition and reaction mechanisms of cyanase remain unclear. Here, crystal structures of cyanase from Escherichia coli were determined in the native form and in complexes with cyanate, bicarbonate and intermediates at 1.5–1.9 Å resolution using synchrotron X-rays and an X-ray free-electron laser. Cyanate and bicarbonate interact with the highly conserved Arg96, Ser122 and Ala123 in the active site. In the presence of a mixture of cyanate and bicarbonate, three different electron densities for intermediates were observed in the cyanase structures. Moreover, the observed electron density could explain the dynamics of the substrate or product. In addition to conformational changes in the substrate-binding pocket, dynamic movement of Leu151 was observed, which functions as a gate for the passage of substrates or products. These findings provide a structural mechanism for the substrate-binding and reaction process of cyanase.
中文翻译:

大肠杆菌氰化酶的结构机制
氰酶在氰酸盐的解毒中起着至关重要的作用,并通过在碳酸氢盐依赖性反应中将氰酸盐转化为氨和二氧化碳,为土壤微生物提供连续的氮源。氰酶与二价阴离子抑制剂复合的结构与生化研究相结合,表明了假定的底物结合位点。然而,氰酶的底物识别和反应机制仍不清楚。在这里,使用同步加速器 X 射线和 X 射线自由电子激光以 1.5–1.9 Å 的分辨率测定了大肠杆菌氰酶的天然形式以及与氰酸盐、碳酸氢盐和中间体的复合物的晶体结构。氰酸盐和碳酸氢盐与活性位点中高度保守的 Arg96、Ser122 和 Ala123 相互作用。在氰酸盐和碳酸氢盐混合物存在的情况下,在氰酶结构中观察到中间体的三种不同电子密度。此外,观察到的电子密度可以解释基材或产品的动力学。除了底物结合袋的构象变化外,还观察到 Leu151 的动态运动,其充当底物或产物通过的大门。这些发现为氰酶的底物结合和反应过程提供了结构机制。
更新日期:2023-11-16
中文翻译:

大肠杆菌氰化酶的结构机制
氰酶在氰酸盐的解毒中起着至关重要的作用,并通过在碳酸氢盐依赖性反应中将氰酸盐转化为氨和二氧化碳,为土壤微生物提供连续的氮源。氰酶与二价阴离子抑制剂复合的结构与生化研究相结合,表明了假定的底物结合位点。然而,氰酶的底物识别和反应机制仍不清楚。在这里,使用同步加速器 X 射线和 X 射线自由电子激光以 1.5–1.9 Å 的分辨率测定了大肠杆菌氰酶的天然形式以及与氰酸盐、碳酸氢盐和中间体的复合物的晶体结构。氰酸盐和碳酸氢盐与活性位点中高度保守的 Arg96、Ser122 和 Ala123 相互作用。在氰酸盐和碳酸氢盐混合物存在的情况下,在氰酶结构中观察到中间体的三种不同电子密度。此外,观察到的电子密度可以解释基材或产品的动力学。除了底物结合袋的构象变化外,还观察到 Leu151 的动态运动,其充当底物或产物通过的大门。这些发现为氰酶的底物结合和反应过程提供了结构机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号