Cancer Treatment Reviews ( IF 11.8 ) Pub Date : 2023-10-31 , DOI: 10.1016/j.ctrv.2023.102650 Lorena Incorvaia 1 , Alessandro Perez 1 , Claudia Marchetti 2 , Chiara Brando 1 , Valerio Gristina 1 , Daniela Cancelliere 1 , Alessia Pivetti 1 , Silvia Contino 1 , Emilia Di Giovanni 1 , Nadia Barraco 1 , Marco Bono 1 , Ambra Giurintano 1 , Tancredi Didier Bazan Russo 1 , Andrea Gottardo 1 , Sofia Cutaia 1 , Erika Pedone 1 , Marta Peri 1 , Lidia Rita Corsini 1 , Daniele Fanale 1 , Antonio Galvano 1 , Giovanni Scambia 2 , Giuseppe Badalamenti 1 , Antonio Russo 1 , Viviana Bazan 3
|
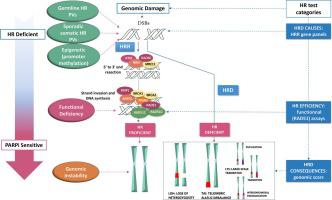
Breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) deleterious variants were the first and, still today, the main biomarkers of poly(ADP)ribose polymerase (PARP)-inhibitors (PARPis) benefit. The recent, increased, numbers of individuals referred for counseling and multigene panel testing, and the remarkable expansion of approved PARPis, not restricted to BRCA1/BRCA2-Pathogenic Variants (PVs), produced a strong clinical need for non-BRCA biomarkers.
Significant limitations of the current testing and assays exist. The different approaches that identify the causes of Homologous Recombination Deficiency (HRD), such as the germline and somatic Homologous Recombination Repair (HRR) gene PVs, the testing showing its consequences, such as the genomic scars, or the novel functional assays such as the RAD51 foci testing, are not interchangeable, and should not be considered as substitutes for each other in clinical practice for guiding use of PARPi in non-BRCA, HRD-associated tumors. Today, the deeper knowledge on the significant relationship among all proteins involved in the HRR, not limited to BRCA, expands the possibility of a successful non-BRCA, HRD-PARPi synthetic lethality and, at the same time, reinforces the need for enhanced definition of HRD biomarkers predicting the magnitude of PARPi benefit.
中文翻译:

治疗诊断生物标志物和 PARP 抑制剂对非 BRCA 相关同源重组缺陷肿瘤患者的有效性:还在透过肮脏的玻璃窗观察吗?
乳腺癌易感基因 1 ( BRCA1 ) 和乳腺癌易感基因 2 ( BRCA2 ) 有害变异是最早的、至今仍是聚 (ADP) 核糖聚合酶 (PARP) 抑制剂 (PARPis) 获益的主要生物标志物。最近,接受咨询和多基因小组测试的人数不断增加,以及批准的 PARPis 的显着扩展(不限于BRCA1/BRCA2致病变异 (PV)),产生了对非BRCA生物标志物的强烈临床需求。
当前的测试和分析存在显着的局限性。识别同源重组缺陷 (HRD) 原因的不同方法,例如种系和体细胞同源重组修复 (HRR) 基因 PV,显示其后果的测试,例如基因组疤痕,或新颖的功能测定,例如RAD51 病灶检测不可互换,在临床实践中不应被视为彼此替代,以指导 PARPi 在非BRCA、HRD 相关肿瘤中的使用。如今,对涉及 HRR 的所有蛋白质(不仅限于BRCA)之间的重要关系有了更深入的了解,扩大了成功实现非BRCA 、HRD-PARPi 合成致死性的可能性,同时也增强了对增强定义的需求HRD 生物标志物预测 PARPi 益处的程度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号