当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Hydrogen Peroxide Formation on Sprayed Water Microdroplets
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-09 , DOI: 10.1021/jacs.3c04643 Agustín J Colussi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-09 , DOI: 10.1021/jacs.3c04643 Agustín J Colussi 1
Affiliation
|
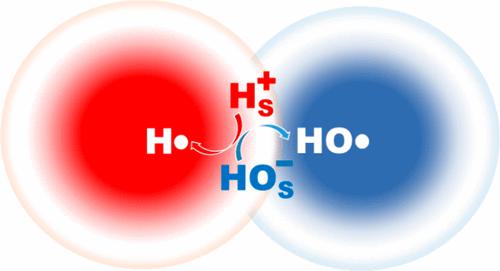
How is H2O2 formed in sprayed water is not well understood. It is believed to involve the association of HO• radicals spontaneously generated from HO– ions by internal electric fields on the surface of neutral microdroplets. Spraying water actually creates charged microdroplets carrying either excess OH– or H+ intrinsic ions that repel each other toward the very surface. The requisite electron transfer (ET) takes place between surface-bound ions: HOS– + HS+ = HOS• + HS•, during encounters between positive and negative microdroplets. The ET endothermicity in bulk water (ΔH = 448 kJ mol–1) is reversed in low-density surface water by the destabilization of the strongly hydrated reactant ions: ΔHhydration(H+ + OH–) = −1670 kJ mol–1, relative to neutral radical products: ΔHhydration(HO• + H•) = −58 kJ mol–1. The formation of H2O2 is driven by the energy supplied for spraying water, and caused by restricted hydration on microdroplet surfaces.
中文翻译:

喷雾水微滴形成过氧化氢的机理
H 2 O 2在喷射水中如何形成尚不清楚。据信,它涉及通过中性微滴表面的内部电场从HO -离子自发产生的HO •自由基的缔合。喷水实际上会产生带电微滴,这些微滴携带过量的 OH -或 H +固有离子,这些离子在表面相互排斥。在正微滴和负微滴相遇期间,必要的电子转移(ET)发生在表面结合离子之间:HO S – + H S + = HO S • + H S • 。散装水中的 ET 吸热性 (Δ H = 448 kJ mol –1 ) 在低密度地表水中通过强水合反应物离子的不稳定而逆转: Δ H水合(H + + OH – ) = −1670 kJ mol – 1,相对于中性自由基产物:ΔH水合( HO • + H • ) = -58 kJ mol –1。H 2 O 2的形成是由喷水所提供的能量驱动的,并且是由微滴表面上的水合作用受限引起的。
更新日期:2023-06-09
中文翻译:

喷雾水微滴形成过氧化氢的机理
H 2 O 2在喷射水中如何形成尚不清楚。据信,它涉及通过中性微滴表面的内部电场从HO -离子自发产生的HO •自由基的缔合。喷水实际上会产生带电微滴,这些微滴携带过量的 OH -或 H +固有离子,这些离子在表面相互排斥。在正微滴和负微滴相遇期间,必要的电子转移(ET)发生在表面结合离子之间:HO S – + H S + = HO S • + H S • 。散装水中的 ET 吸热性 (Δ H = 448 kJ mol –1 ) 在低密度地表水中通过强水合反应物离子的不稳定而逆转: Δ H水合(H + + OH – ) = −1670 kJ mol – 1,相对于中性自由基产物:ΔH水合( HO • + H • ) = -58 kJ mol –1。H 2 O 2的形成是由喷水所提供的能量驱动的,并且是由微滴表面上的水合作用受限引起的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号