当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A general catalytic synthetic strategy for highly strained methylenecyclobutanes and spiromethylenecyclobutanes
Chemical Science ( IF 7.6 ) Pub Date : 2023-06-09 , DOI: 10.1039/d3sc01103h Haotian Zhao 1 , Yu Lin 1 , Mingyu Jiang 1 , Bo Su 1
Chemical Science ( IF 7.6 ) Pub Date : 2023-06-09 , DOI: 10.1039/d3sc01103h Haotian Zhao 1 , Yu Lin 1 , Mingyu Jiang 1 , Bo Su 1
Affiliation
|
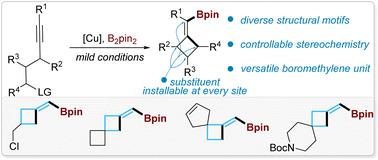
Highly strained methylenecyclobutanes (MCBs) are intriguing scaffolds in synthetic chemistry and drug discovery, but there is no such strategy that enables the synthesis of structurally diverse MCBs with defined stereochemistry. We report a general synthetic strategy for (boromethylene)cyclobutanes (BMCBs) and spiro-BMCBs by a challenging Cu-catalyzed highly chemo-, stereo-, and regioselective borylative cyclization of aliphatic alkynes. This strategy not only enables the installation of various functionalities at each site on the MCB skeleton with unambiguous stereochemistry but also introduces a versatile boromethylene unit that is readily transformable to a wide range of new functional groups; these features significantly expand the structural diversity of MCBs and are particularly valuable in drug discovery. The concise and divergent total syntheses of four cyclobutane-containing natural products were achieved from one common BMCB obtained by this strategy. The origin of the high regioselectivity in the borylcupration of alkynes and the high efficiency of the strained ring cyclization was also studied.
中文翻译:

高张力亚甲基环丁烷和螺亚甲基环丁烷的通用催化合成策略
高张力亚甲基环丁烷 (MCB) 是合成化学和药物发现中令人感兴趣的支架,但没有这样的策略能够合成具有明确立体化学的结构多样的 MCB。我们报告了通过具有挑战性的铜催化的脂肪族炔烃的高度化学、立体和区域选择性硼基环化来合成(硼甲基)环丁烷(BMCB)和螺环-BMCB 的一般合成策略。这种策略不仅能够在 MCB 骨架上的每个位点安装具有明确立体化学的各种功能,而且还引入了一种多功能的硼亚甲基单元,该单元很容易转化为各种新的官能团;这些特征显着扩大了 MCB 的结构多样性,在药物发现中特别有价值。利用该策略获得的一种常见的BMCB,实现了四种含环丁烷天然产物的简洁而发散的全合成。还研究了炔烃硼基化的高区域选择性和高张力环环化效率的起源。
更新日期:2023-06-09
中文翻译:

高张力亚甲基环丁烷和螺亚甲基环丁烷的通用催化合成策略
高张力亚甲基环丁烷 (MCB) 是合成化学和药物发现中令人感兴趣的支架,但没有这样的策略能够合成具有明确立体化学的结构多样的 MCB。我们报告了通过具有挑战性的铜催化的脂肪族炔烃的高度化学、立体和区域选择性硼基环化来合成(硼甲基)环丁烷(BMCB)和螺环-BMCB 的一般合成策略。这种策略不仅能够在 MCB 骨架上的每个位点安装具有明确立体化学的各种功能,而且还引入了一种多功能的硼亚甲基单元,该单元很容易转化为各种新的官能团;这些特征显着扩大了 MCB 的结构多样性,在药物发现中特别有价值。利用该策略获得的一种常见的BMCB,实现了四种含环丁烷天然产物的简洁而发散的全合成。还研究了炔烃硼基化的高区域选择性和高张力环环化效率的起源。









































 京公网安备 11010802027423号
京公网安备 11010802027423号