当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effect of S-alkylation on organocatalytic enamine activation through imidazolidine-4-thiones
Chemical Communications ( IF 4.3 ) Pub Date : 2023-06-09 , DOI: 10.1039/d3cc01912h Magenta J Hensinger 1 , Anna C Closs 1, 2 , Oliver Trapp 1, 2 , Armin R Ofial 1
Chemical Communications ( IF 4.3 ) Pub Date : 2023-06-09 , DOI: 10.1039/d3cc01912h Magenta J Hensinger 1 , Anna C Closs 1, 2 , Oliver Trapp 1, 2 , Armin R Ofial 1
Affiliation
|
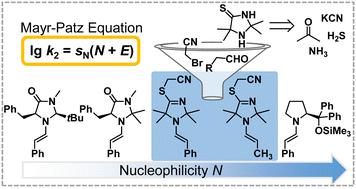
Imidazolidine-4-thiones have been suggested as potential prebiotic organocatalysts for light-driven α-alkylations of aldehydes by bromoacetonitrile. However, imidazolidine-4-thiones react with bromoacetonitrile to give S-cyanomethylated dihydroimidazoles. Kinetic studies show that enamines derived from these cyclic secondary amines and aldehydes are more nucleophilic than enamines derived from aldehydes and MacMillan organocatalysts.
中文翻译:

S-烷基化对咪唑烷-4-硫酮有机催化烯胺活化的影响
咪唑烷-4-硫酮已被建议作为潜在的益生元有机催化剂,用于光驱动的溴乙腈对醛的 α-烷基化。然而,咪唑烷-4-硫酮与溴乙腈反应生成S-氰甲基化二氢咪唑。动力学研究表明,源自这些环状仲胺和醛的烯胺比源自醛和麦克米伦有机催化剂的烯胺更具亲核性。
更新日期:2023-06-09
中文翻译:

S-烷基化对咪唑烷-4-硫酮有机催化烯胺活化的影响
咪唑烷-4-硫酮已被建议作为潜在的益生元有机催化剂,用于光驱动的溴乙腈对醛的 α-烷基化。然而,咪唑烷-4-硫酮与溴乙腈反应生成S-氰甲基化二氢咪唑。动力学研究表明,源自这些环状仲胺和醛的烯胺比源自醛和麦克米伦有机催化剂的烯胺更具亲核性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号